How to electroplate metal
How To Electroplate Metal. Dissolve five tablespoons of table sugar and 3 1 2 tablespoons of epsom salts in the container with the vinegar in it. It is essential to do this step well for an even electroplate. Engineers use controlled electrolysis to transfer the desired metal coating from an anode a part containing the metal that will be used as the plating to a cathode the part to be plated. Electroplating gives us the ability to use common or garden electricity a sacrificial metal and a few cheap and readily available chemicals to transform an everyday metal such as copper or steel with a thin layer of another much more desirable metal such as chrome gold or even platinum.
 Electroplating Wikipedia From en.wikipedia.org
Electroplating Wikipedia From en.wikipedia.org
Engineers use controlled electrolysis to transfer the desired metal coating from an anode a part containing the metal that will be used as the plating to a cathode the part to be plated. The longer you expose your product to the current the thicker the layer of plating will be. How electroplating works. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure. A cell consists of two electrodes conductors usually made of metal which are held apart from one another.
Before starting the electroplating method you must ensure that the metal is clean so the new metal atoms can form a solid bond to the recipient metal.
In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. For different metals different temperatures voltage levels and immersion times are variables to consider. Electroplating is the process of using electrodeposition to coat an object in a layer of metal s. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Dissolve five tablespoons of table sugar and 3 1 2 tablespoons of epsom salts in the container with the vinegar in it. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure.
 Source: wikihow.com
Source: wikihow.com
To assemble an electroplating system you need two metal objects a container an electrolyte like salt water some wire and a power supply. Electroplating is the process of using electrodeposition to coat an object in a layer of metal s. The deposition begins as soon as you turn on the electrical current. The electrodes are immersed in an electrolyte a solution. In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery.
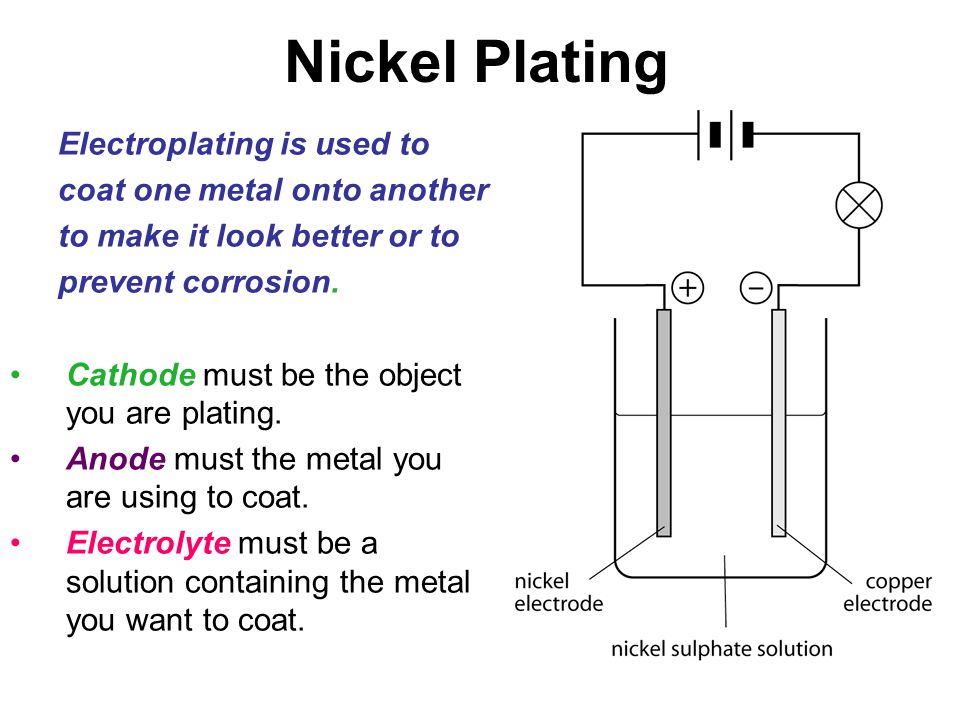 Source: slideplayer.com
Source: slideplayer.com
Generally cleaning is done by dipping the electrode into a strong acid or alkaline solution or by briefly connecting the electroplating circuit in reverse. Clean the metal to be electroplated. Strip about a quarter inch from each wire. The result of this is metal deposition. A silver finish is used in almost all industries and cannot be substituted by any other metal due to its properties.
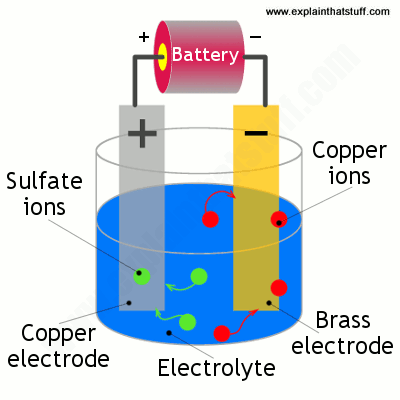 Source: explainthatstuff.com
Source: explainthatstuff.com
A cell consists of two electrodes conductors usually made of metal which are held apart from one another. The longer you expose your product to the current the thicker the layer of plating will be. For different metals different temperatures voltage levels and immersion times are variables to consider. In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. Before starting the electroplating method you must ensure that the metal is clean so the new metal atoms can form a solid bond to the recipient metal.
 Source: technologystudent.com
Source: technologystudent.com
Before starting the electroplating method you must ensure that the metal is clean so the new metal atoms can form a solid bond to the recipient metal. Clean the metal to be electroplated. For electroplating to occur the plating solution must contain metal ions of the metal you want to plate. Pull the two wires apart and cut one of the wires two or three inches shorter than the other this will help prevent you from accidentally shorting wires together later. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure.
 Source: cikguwong.blogspot.com
Source: cikguwong.blogspot.com
In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. Dissolve five tablespoons of table sugar and 3 1 2 tablespoons of epsom salts in the container with the vinegar in it. In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. Strip about a quarter inch from each wire. Wall warts the ugly black things that come with some electronics cut the barrel jack off of the end of your dc power supply.
 Source: pinterest.com
Source: pinterest.com
It is essential to do this step well for an even electroplate. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure. A cell consists of two electrodes conductors usually made of metal which are held apart from one another. The electrodes are immersed in an electrolyte a solution. Engineers use controlled electrolysis to transfer the desired metal coating from an anode a part containing the metal that will be used as the plating to a cathode the part to be plated.
 Source: cdtusa.net
Source: cdtusa.net
The longer you expose your product to the current the thicker the layer of plating will be. To assemble an electroplating system you need two metal objects a container an electrolyte like salt water some wire and a power supply. In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure. The longer you expose your product to the current the thicker the layer of plating will be.
 Source: m.youtube.com
Source: m.youtube.com
Dissolve five tablespoons of table sugar and 3 1 2 tablespoons of epsom salts in the container with the vinegar in it. Wall warts the ugly black things that come with some electronics cut the barrel jack off of the end of your dc power supply. To assemble an electroplating system you need two metal objects a container an electrolyte like salt water some wire and a power supply. It is essential to do this step well for an even electroplate. The electrodes are immersed in an electrolyte a solution.
 Source: sciencestruck.com
Source: sciencestruck.com
If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure. Silver plating has been used since the invention of coins and metal smithing and has the most applications compared to any other types of plated metal. Wall warts the ugly black things that come with some electronics cut the barrel jack off of the end of your dc power supply. Strip about a quarter inch from each wire. Clean the metal to be electroplated.
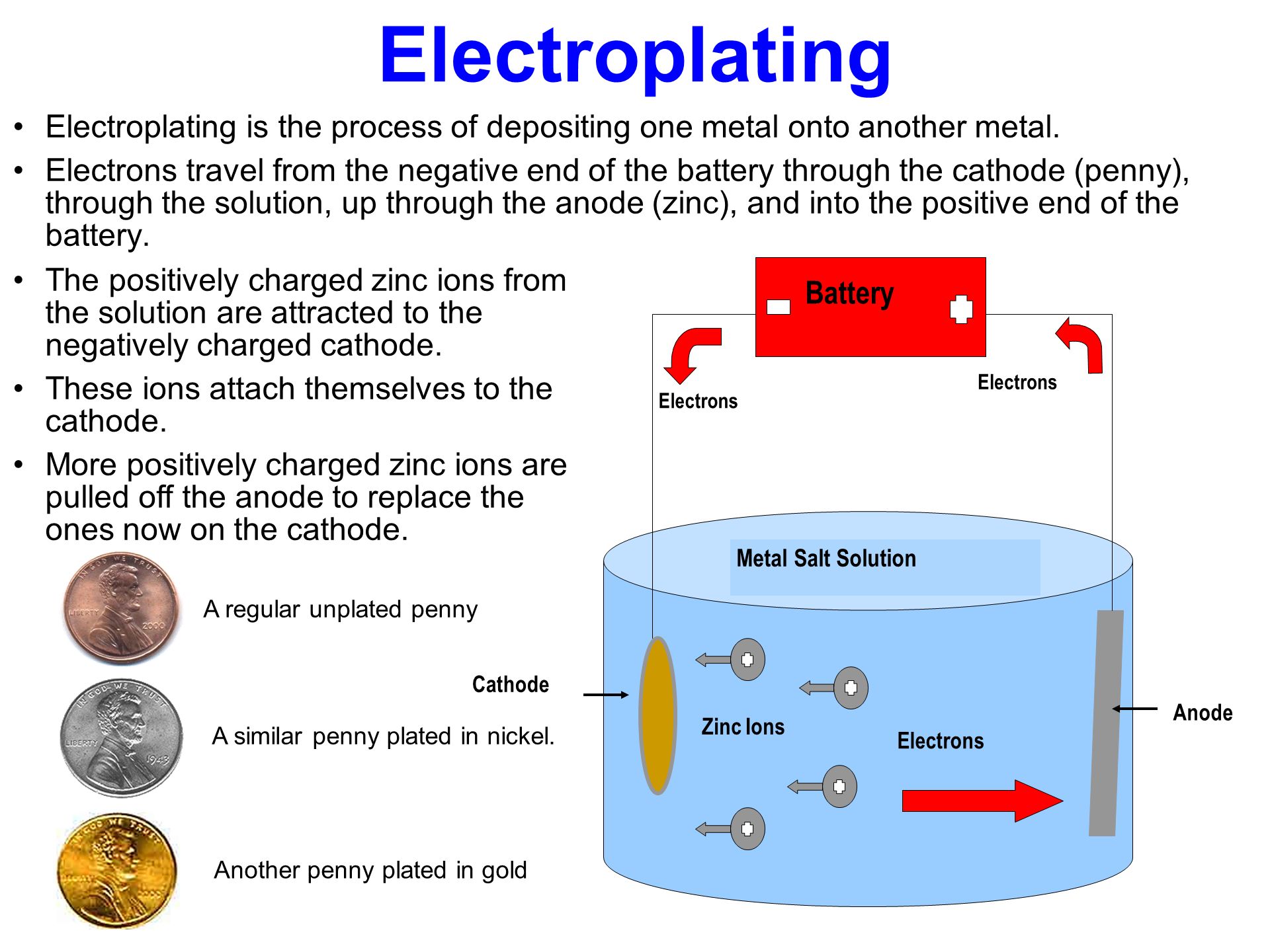 Source: slideplayer.com
Source: slideplayer.com
Wear gloves to both protect your hands and keep the metal surface clean. How electroplating works. The deposition begins as soon as you turn on the electrical current. Electroplating is the process of using electrodeposition to coat an object in a layer of metal s. For different metals different temperatures voltage levels and immersion times are variables to consider.
 Source: thoughtco.com
Source: thoughtco.com
In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. Generally cleaning is done by dipping the electrode into a strong acid or alkaline solution or by briefly connecting the electroplating circuit in reverse. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Before starting the electroplating method you must ensure that the metal is clean so the new metal atoms can form a solid bond to the recipient metal. Engineers use controlled electrolysis to transfer the desired metal coating from an anode a part containing the metal that will be used as the plating to a cathode the part to be plated.
 Source: instructables.com
Source: instructables.com
Pull the two wires apart and cut one of the wires two or three inches shorter than the other this will help prevent you from accidentally shorting wires together later. For different metals different temperatures voltage levels and immersion times are variables to consider. The deposition begins as soon as you turn on the electrical current. Wall warts the ugly black things that come with some electronics cut the barrel jack off of the end of your dc power supply. Electroplating is the process of using electrodeposition to coat an object in a layer of metal s.
 Source: wikihow.com
Source: wikihow.com
Wear gloves to both protect your hands and keep the metal surface clean. Silver plating has been used since the invention of coins and metal smithing and has the most applications compared to any other types of plated metal. The longer you expose your product to the current the thicker the layer of plating will be. Allow the zinc to remain in the vinegar for at least 15 minutes before beginning the electroplating. Generally cleaning is done by dipping the electrode into a strong acid or alkaline solution or by briefly connecting the electroplating circuit in reverse.
 Source: m.youtube.com
Source: m.youtube.com
The electrodes are immersed in an electrolyte a solution. In the example in the picture there is a bucket of salt water a horseshoe that has been cleaned a wire the goes between the horseshoe and one end of the battery and a wire that goes between a chunk of zinc and the other end of the battery. The deposition begins as soon as you turn on the electrical current. Pull the two wires apart and cut one of the wires two or three inches shorter than the other this will help prevent you from accidentally shorting wires together later. The electrodes are immersed in an electrolyte a solution.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Wall warts the ugly black things that come with some electronics cut the barrel jack off of the end of your dc power supply. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. How electroplating works. The longer you expose your product to the current the thicker the layer of plating will be. If the electrode is really clean atoms from the plating metal bond to it effectively by joining very strongly onto the outside edges of its crystalline structure.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to electroplate metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





