How to electroplate copper
How To Electroplate Copper. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. Your solution should be dark blue. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. Use one alligator clip to attach the copper electrode to the positive terminal of the battery this is now the anode and the other to attach the key to the negative terminal now called the cathode.

Your solution should be dark blue. You now have a copper ion solution that can be used for electroplating. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. Use one alligator clip to attach the copper electrode to the positive terminal of the battery this is now the anode and the other to attach the key to the negative terminal now called the cathode. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. Submerge the wafer to be plated into an electrolyte bath apply a current and copper ions will migrate and deposit onto regions with a pre existing metal seed layer.
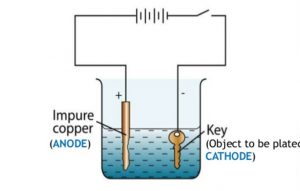
Into a container of water are placed a copper rod and the item.
The solution will turn blue indicating that the scrub has dissolved into the solution. Stir copper sulfate into some hot water in a beaker until no more will dissolve. This means that the iron electrode is the cathode and the copper electrode is the anode add the copper sulfate solution to the bath until the electrodes are just covered close the switch. You can substitute the copper scoring pads for scrap copper pipe or wire. Wear gloves from this step on because the copper solution is toxic. How electroplating works that will give you easy instructions for the project.
 Source: m.youtube.com
Source: m.youtube.com
Stir copper sulfate into some hot water in a beaker until no more will dissolve. Into a container of water are placed a copper rod and the item. Submerge the wafer to be plated into an electrolyte bath apply a current and copper ions will migrate and deposit onto regions with a pre existing metal seed layer. Both metals copper and nickel gets dissolved in the acid into positive ions cu 2 or cu based on your acid coin condition and ni 2. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on.
 Source: researchgate.net
Source: researchgate.net
But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. Both metals copper and nickel gets dissolved in the acid into positive ions cu 2 or cu based on your acid coin condition and ni 2. You can substitute the copper scoring pads for scrap copper pipe or wire. How electroplating works that will give you easy instructions for the project.
 Source: m.youtube.com
Source: m.youtube.com
Your solution should be dark blue. Connect the iron electrode to the battery negative end and the copper electrode to the switch positive end. Here is how it works. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. You now have a copper ion solution that can be used for electroplating.
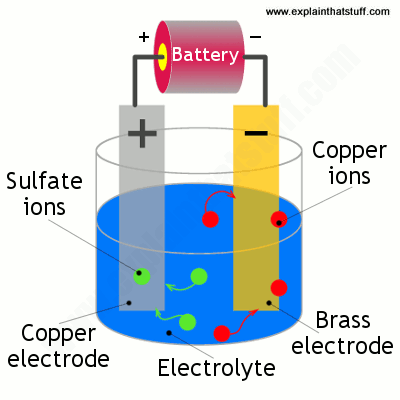 Source: explainthatstuff.com
Source: explainthatstuff.com
Soak half of the copper scrubbing pad in the vinegar peroxide solution. You now have a copper ion solution that can be used for electroplating. Since cu 2 and cu both have a higher or more positive reduction potential than ni 2 which actually have a negative reduction potential cu 2 or cu will be more attracted to accept electrons from the negative terminal which is your job. The solution will turn blue indicating that the scrub has dissolved into the solution. Fill the bowl with copper sulfate solution until both metals and exposed wire ends are completely submerged.

Connect the loose end of the black wire to the negative terminal of a 9 volt battery and the red wire to the positive terminal. Since cu 2 and cu both have a higher or more positive reduction potential than ni 2 which actually have a negative reduction potential cu 2 or cu will be more attracted to accept electrons from the negative terminal which is your job. Into a container of water are placed a copper rod and the item. Submerge the wafer to be plated into an electrolyte bath apply a current and copper ions will migrate and deposit onto regions with a pre existing metal seed layer. Here is how it works.
 Source: slideplayer.com
Source: slideplayer.com
Connect the iron electrode to the battery negative end and the copper electrode to the switch positive end. Submerge the wafer to be plated into an electrolyte bath apply a current and copper ions will migrate and deposit onto regions with a pre existing metal seed layer. Here is how it works. Copper electroplating requires electricty. Both metals copper and nickel gets dissolved in the acid into positive ions cu 2 or cu based on your acid coin condition and ni 2.
 Source: brainly.in
Source: brainly.in
But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. Since cu 2 and cu both have a higher or more positive reduction potential than ni 2 which actually have a negative reduction potential cu 2 or cu will be more attracted to accept electrons from the negative terminal which is your job. The concept of copper electroplating is straightforward. How electroplating works that will give you easy instructions for the project. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item.
 Source: pinterest.cl
Source: pinterest.cl
The concept of copper electroplating is straightforward. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. Wear gloves from this step on because the copper solution is toxic. Soak half of the copper scrubbing pad in the vinegar peroxide solution. Your solution should be dark blue.
 Source: chemedx.org
Source: chemedx.org
It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. If you or a reader wish to demo copper plating for a school science project we have an faq. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Copper electroplating requires electricty. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers.
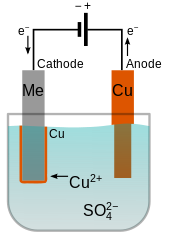 Source: en.wikipedia.org
Source: en.wikipedia.org
You now have a copper ion solution that can be used for electroplating. But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. Soak half of the copper scrubbing pad in the vinegar peroxide solution. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item.
 Source: student-sc.blogspot.com
Source: student-sc.blogspot.com
Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. This means that the iron electrode is the cathode and the copper electrode is the anode add the copper sulfate solution to the bath until the electrodes are just covered close the switch. Soak half of the copper scrubbing pad in the vinegar peroxide solution. The solution will turn blue indicating that the scrub has dissolved into the solution. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item.
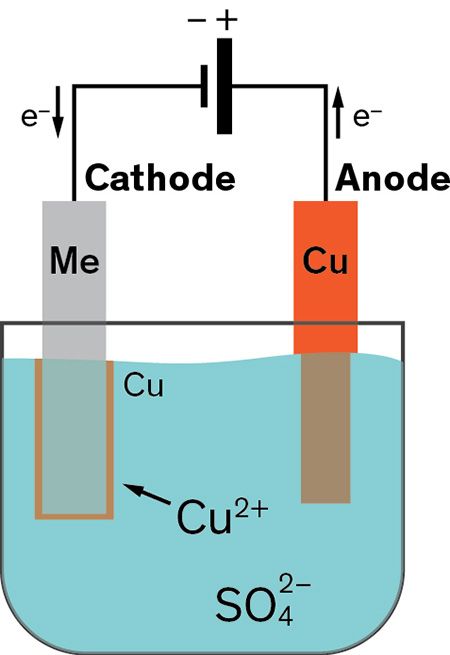
Submerge the wafer to be plated into an electrolyte bath apply a current and copper ions will migrate and deposit onto regions with a pre existing metal seed layer. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. You can substitute the copper scoring pads for scrap copper pipe or wire. The concept of copper electroplating is straightforward. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item.
 Source: yenka.com
Source: yenka.com
Both metals copper and nickel gets dissolved in the acid into positive ions cu 2 or cu based on your acid coin condition and ni 2. But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. The concept of copper electroplating is straightforward. Your solution should be dark blue. You now have a copper ion solution that can be used for electroplating.
 Source: classnotes.org.in
Source: classnotes.org.in
If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. Copper electroplating requires electricty. But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item.
 Source: quora.com
Source: quora.com
Connect the iron electrode to the battery negative end and the copper electrode to the switch positive end. The solution will turn blue indicating that the scrub has dissolved into the solution. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. You now have a copper ion solution that can be used for electroplating. Clip the copper plating metal against the exposed end of the red wire and the aluminum metal to the black wire.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to electroplate copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.