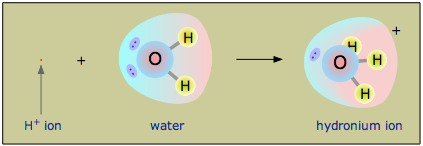
How to electrolyze water
How To Electrolyze Water. For an easy shortcut make a base mixture of all your ingredients except the water up to 1 day ahead. Pour your electrolyte water into a glass over ice for a hydrating post workout treat. The electrolysis of water. How electrolyzed water works.
 Hydrogen Production By Pem Water Electrolysis A Review Sciencedirect From sciencedirect.com
Hydrogen Production By Pem Water Electrolysis A Review Sciencedirect From sciencedirect.com
Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. If you add salt the reaction will be different than just electrolyzing pure water. Stir until the powder is fully dissolved. The electrolysis of water. Water can be decomposed by passing an electric current through it. Add a cup of warm water to your glass and a tablespoon of salt or baking soda.
How electrolyzed water works.
Here s how it works. The electrolysis of water. Add a cup of warm water to your glass and a tablespoon of salt or baking soda. Pour your electrolyte water into a glass over ice for a hydrating post workout treat. Solution for it is planned to electrolyze the water according to the following reaction to produce 30 liters of hydrogen gas at 25 c and 1 atm. At one electrode called the cathode electrons pass into the solution and cause areduction.
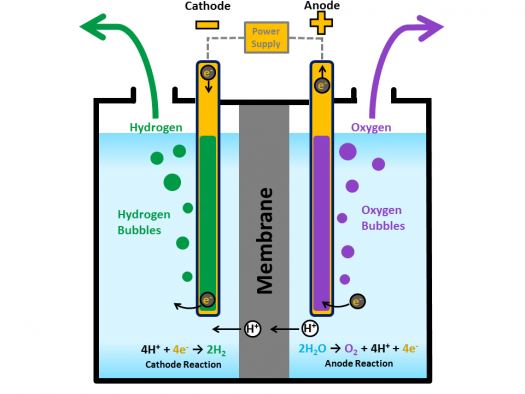 Source: energy.gov
Source: energy.gov
Water can be decomposed by passing an electric current through it. Your homemade electrolyte water will keep in the fridge for up to 2 days. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. At one electrode called the cathode electrons pass into the solution and cause areduction. Solution for it is planned to electrolyze the water according to the following reaction to produce 30 liters of hydrogen gas at 25 c and 1 atm.
 Source: sciencedirect.com
Source: sciencedirect.com
By adding salt or baking soda to the water you increase the conduction of electricity through the water. Stir until the powder is fully dissolved. If you add salt the reaction will be different than just electrolyzing pure water. For an easy shortcut make a base mixture of all your ingredients except the water up to 1 day ahead. Chloride ions form hypochlorous acid hclo which is a powerful disinfectant.
 Source: sciencedirect.com
Source: sciencedirect.com
If you add salt the reaction will be different than just electrolyzing pure water. Your homemade electrolyte water will keep in the fridge for up to 2 days. The electrolysis of water. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. By adding salt or baking soda to the water you increase the conduction of electricity through the water.
Source: en.wikipedia.org
For an easy shortcut make a base mixture of all your ingredients except the water up to 1 day ahead. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Pour your electrolyte water into a glass over ice for a hydrating post workout treat. The electrolysis of water. This recipe creates two servings.
 Source: researchgate.net
Source: researchgate.net
The electrolysis of water. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. Chloride ions form hypochlorous acid hclo which is a powerful disinfectant. By adding salt or baking soda to the water you increase the conduction of electricity through the water.
 Source: researchgate.net
Source: researchgate.net
When thishappens the electrons from the electric current cause an oxidation reductionreaction. Stir until the powder is fully dissolved. Add a cup of warm water to your glass and a tablespoon of salt or baking soda. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. The electrolysis of water.
Source: quora.com
Stir until the powder is fully dissolved. Electrolyzed water is produced by applying a low voltage electrical charge to saltwater. How electrolyzed water works. Add a cup of warm water to your glass and a tablespoon of salt or baking soda. Sodium ions form sodium hydroxide naoh a strong base that cleans much like a detergent.
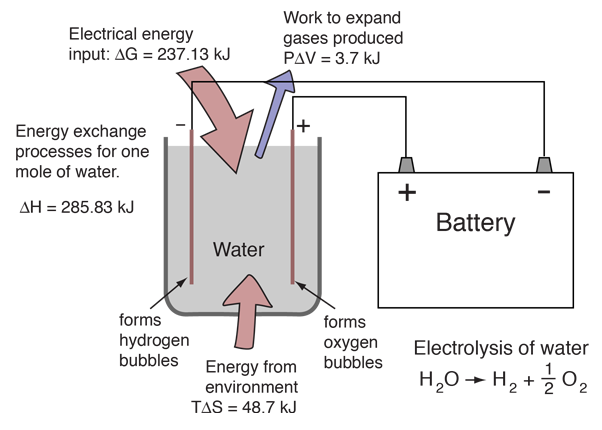 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
Add a cup of warm water to your glass and a tablespoon of salt or baking soda. When thishappens the electrons from the electric current cause an oxidation reductionreaction. Solution for it is planned to electrolyze the water according to the following reaction to produce 30 liters of hydrogen gas at 25 c and 1 atm. Water can be decomposed by passing an electric current through it. By adding salt or baking soda to the water you increase the conduction of electricity through the water.
 Source: instructables.com
Source: instructables.com
How electrolyzed water works. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Your homemade electrolyte water will keep in the fridge for up to 2 days. Stir until the powder is fully dissolved. This recipe creates two servings.
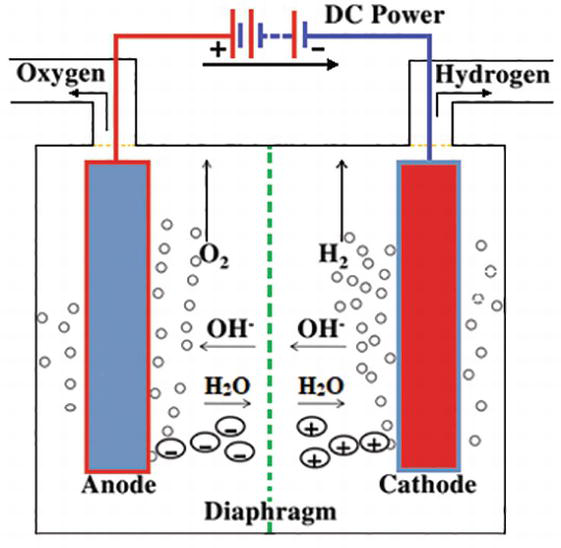 Source: intechopen.com
Source: intechopen.com
The electrolysis of water. Pour your electrolyte water into a glass over ice for a hydrating post workout treat. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts.
 Source: sciencedirect.com
Source: sciencedirect.com
When thishappens the electrons from the electric current cause an oxidation reductionreaction. Solution for it is planned to electrolyze the water according to the following reaction to produce 30 liters of hydrogen gas at 25 c and 1 atm. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. At one electrode called the cathode electrons pass into the solution and cause areduction. This recipe creates two servings.
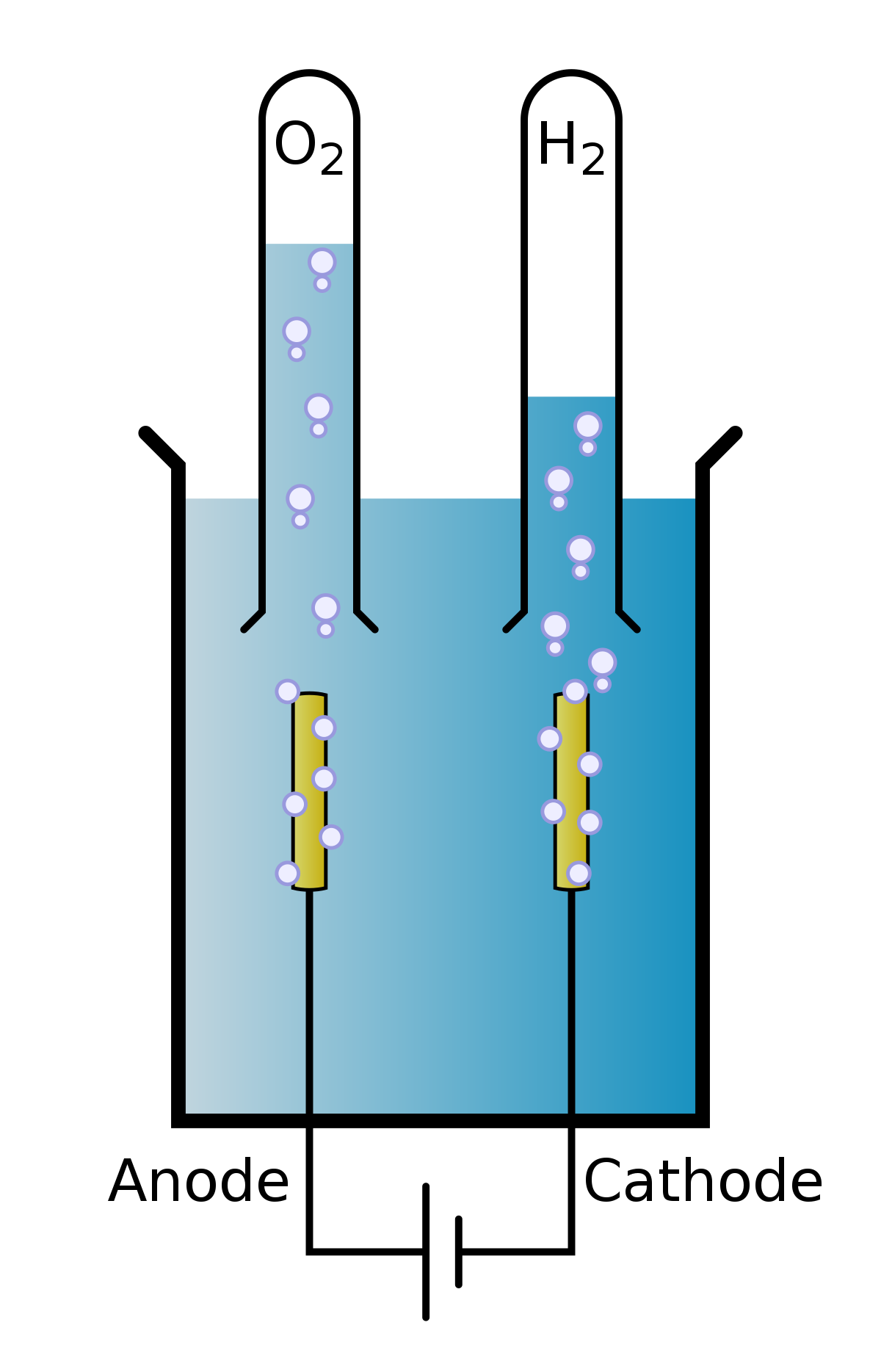 Source: simple.wikipedia.org
Source: simple.wikipedia.org
The electrolysis of water. The electrolysis of water. Add a cup of warm water to your glass and a tablespoon of salt or baking soda. At one electrode called the cathode electrons pass into the solution and cause areduction. Pour your electrolyte water into a glass over ice for a hydrating post workout treat.
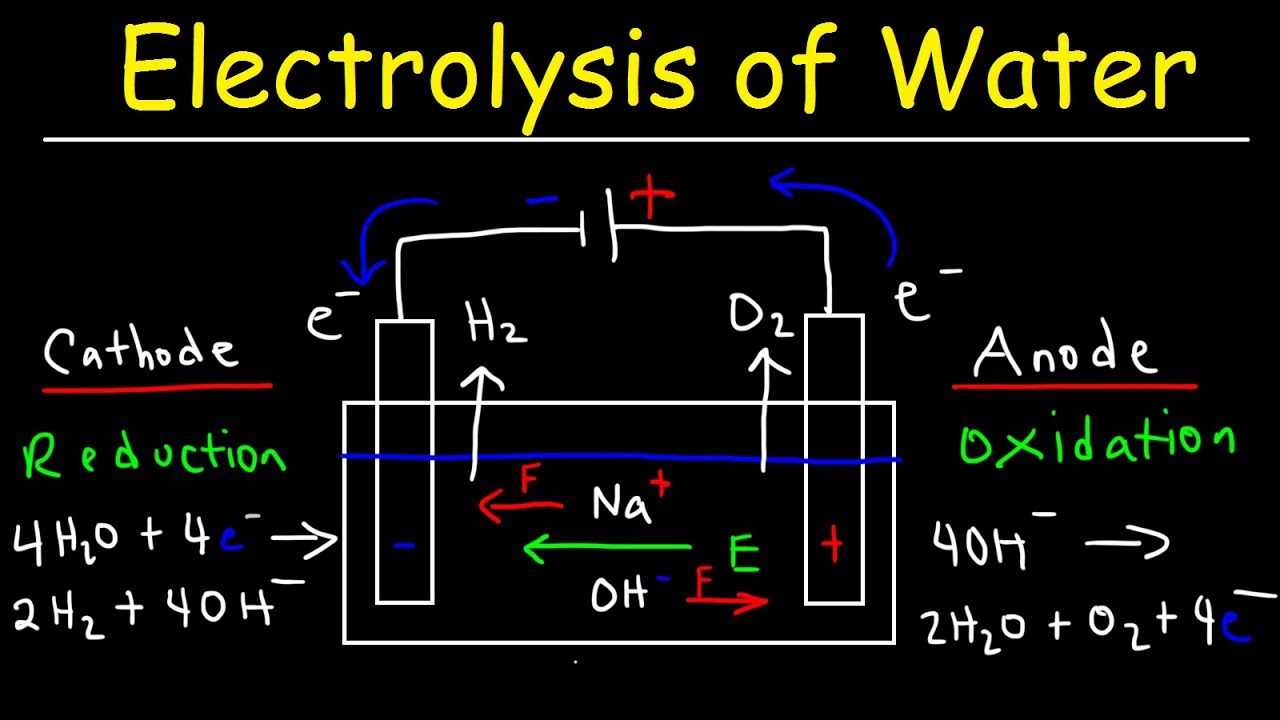 Source: youtube.com
Source: youtube.com
Water can be decomposed by passing an electric current through it. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. Store it in the fridge. For an easy shortcut make a base mixture of all your ingredients except the water up to 1 day ahead. Your homemade electrolyte water will keep in the fridge for up to 2 days.
 Source: pinterest.com
Source: pinterest.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Stir until the powder is fully dissolved. At one electrode called the cathode electrons pass into the solution and cause areduction. The electrolysis of water. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas.
 Source: kidslovekits.com
Source: kidslovekits.com
If you add salt the reaction will be different than just electrolyzing pure water. Sodium ions form sodium hydroxide naoh a strong base that cleans much like a detergent. Water can be decomposed by passing an electric current through it. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. Stir until the powder is fully dissolved.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to electrolyze water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.