How is electroplating done
How Is Electroplating Done. The current is provided by an external power supply. How electroplating works. And the anode is usually either a block of that metal or of some inert conductive material. The electrodes and electrolyte are made from carefully chosen elements or compounds.
 Electroplating Wikipedia From en.wikipedia.org
Electroplating Wikipedia From en.wikipedia.org
The electrodes and electrolyte are made from carefully chosen elements or compounds. The electrolyte is a solution of a salt of the metal to be coated. Jan 05 2021 what is electroplating and how is it done. How electroplating works. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Electroplating is widely used in indus.
The electrodes are immersed in an electrolyte a solution.
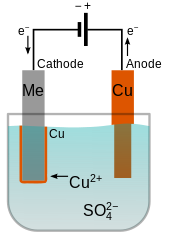
And the anode is usually either a block of that metal or of some inert conductive material. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply. The electrodes are immersed in an electrolyte a solution. This video is highly rated by class 8 students and has been viewed 912 times. The electrodes and electrolyte are made from carefully chosen elements or compounds. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface.
 Source: britannica.com
Source: britannica.com
Electroplating is widely used in indus. This video is highly rated by class 8 students and has been viewed 912 times. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply. The electrolyte is a solution of a salt of the metal to be coated. A cell consists of two electrodes conductors usually made of metal which are held apart from one another.
 Source: byjus.com
Source: byjus.com
Electroplating is widely used in indus. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. The electrolyte is a solution of a salt of the metal to be coated. This video is highly rated by class 8 students and has been viewed 912 times. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply.
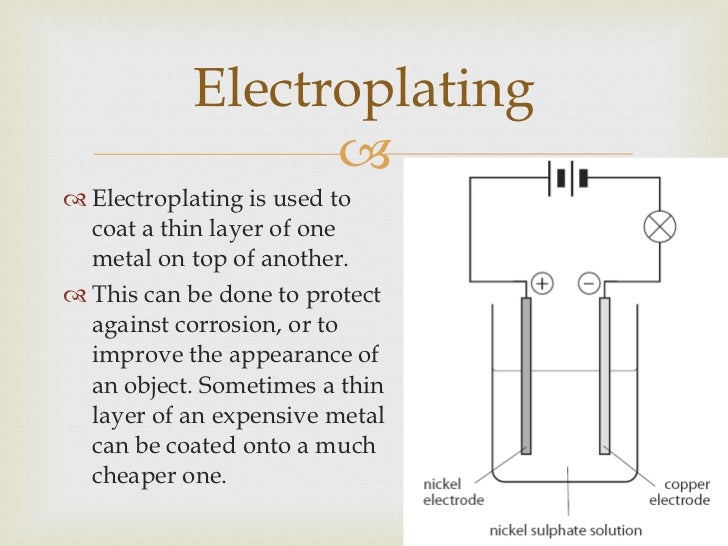 Source: slideshare.net
Source: slideshare.net
How electroplating works. Class 8 video edurev is made by best teachers of class 8. And the anode is usually either a block of that metal or of some inert conductive material. The current is provided by an external power supply. Jan 05 2021 what is electroplating and how is it done.
 Source: quora.com
Source: quora.com
The part to be coated acts as the cathode of an electrolytic cell. Electroplating is widely used in indus. Electroplating involves passing an electric current through a solution called an electrolyte. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. The part to be coated acts as the cathode of an electrolytic cell.
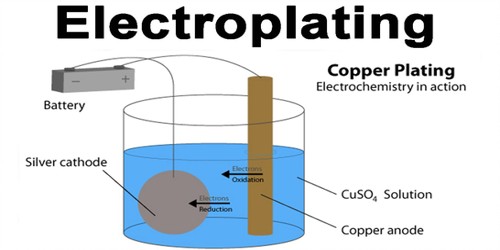 Source: assignmentpoint.com
Source: assignmentpoint.com
This video is highly rated by class 8 students and has been viewed 912 times. This video is highly rated by class 8 students and has been viewed 912 times. The electrodes are immersed in an electrolyte a solution. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface.
 Source: thoughtco.com
Source: thoughtco.com
Class 8 video edurev is made by best teachers of class 8. The electrolyte is a solution of a salt of the metal to be coated. Electroplating is widely used in indus. How electroplating works. The electrodes and electrolyte are made from carefully chosen elements or compounds.
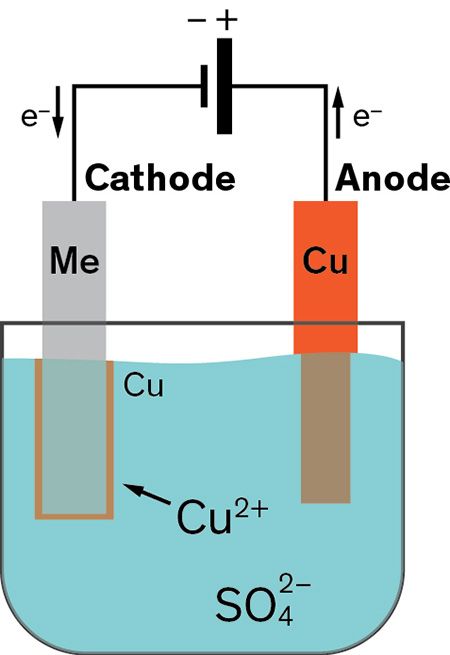
Class 8 video edurev is made by best teachers of class 8. The part to be coated acts as the cathode of an electrolytic cell. Jan 05 2021 what is electroplating and how is it done. The current is provided by an external power supply. How electroplating works.

Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. Electroplating involves passing an electric current through a solution called an electrolyte. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The current is provided by an external power supply. The electrolyte is a solution of a salt of the metal to be coated.
 Source: feechiescience.wordpress.com
Source: feechiescience.wordpress.com
This video is highly rated by class 8 students and has been viewed 912 times. Class 8 video edurev is made by best teachers of class 8. The current is provided by an external power supply. The part to be coated acts as the cathode of an electrolytic cell. And the anode is usually either a block of that metal or of some inert conductive material.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. This video is highly rated by class 8 students and has been viewed 912 times. A cell consists of two electrodes conductors usually made of metal which are held apart from one another. And the anode is usually either a block of that metal or of some inert conductive material. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current.
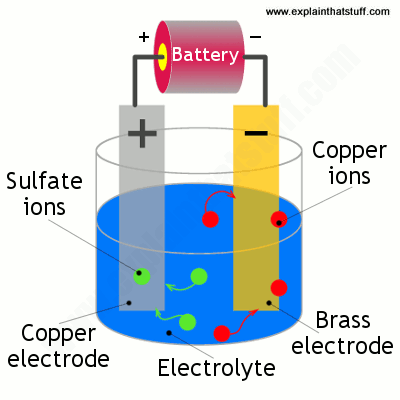 Source: explainthatstuff.com
Source: explainthatstuff.com
Electroplating involves passing an electric current through a solution called an electrolyte. Class 8 video edurev is made by best teachers of class 8. The electrodes and electrolyte are made from carefully chosen elements or compounds. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply. The electrolyte is a solution of a salt of the metal to be coated.
 Source: researchgate.net
Source: researchgate.net
The electrodes and electrolyte are made from carefully chosen elements or compounds. How electroplating works. The electrodes and electrolyte are made from carefully chosen elements or compounds. Electroplating is widely used in indus. This video is highly rated by class 8 students and has been viewed 912 times.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. And the anode is usually either a block of that metal or of some inert conductive material. The part to be coated acts as the cathode of an electrolytic cell. How electroplating works. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current.
 Source: youtube.com
Source: youtube.com
Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Electroplating involves passing an electric current through a solution called an electrolyte. Electroplating is widely used in indus. A cell consists of two electrodes conductors usually made of metal which are held apart from one another. The part to be coated acts as the cathode of an electrolytic cell.

The electrolyte is a solution of a salt of the metal to be coated. The electrodes are immersed in an electrolyte a solution. How electroplating works. Electroplating is widely used in indus. The part to be coated acts as the cathode of an electrolytic cell.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how is electroplating done by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





