How is a water molecule like a magnet
How Is A Water Molecule Like A Magnet. These charged ends can attract other water molecules. Because of the natural polarity water molecules attract one another and stick together forming hydrogen bonds. Which property cohesion or adhesion causes surface tension in water. Water is like a magnet because it has magnetic poles which are attracted to the.
 Magnetism Ppt Download From slideplayer.com
Magnetism Ppt Download From slideplayer.com
Water h 2 o has 2 hydrogen atoms and 1 oxygen atom. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. These charged ends can attract other water molecules. It has partial positive charge on the hydrogen atoms and the partial negative charge on the oxygen atom. The magnetic susceptibility values of various molecular fragments are called pascal s constants. Like a magnet and the earth.
Additionally how is a water molecule polar.
Accordingly how is a water molecule like a magnet quizlet. Water h 2 o has 2 hydrogen atoms and 1 oxygen atom. Is a magnet more dense. Additionally how is a water molecule polar. This forms very weak bonds between molecules called hydrogen bonds that are constantly being broken and reforming. Just like a magnet water molecules attract each other due to their oppositely charged atoms.
 Source: slideplayer.com
Source: slideplayer.com
Diamagnetic materials like water or water based materials have a relative magnetic permeability that is less than or equal to 1 and therefore a magnetic susceptibility less than or equal to 0 since susceptibility is defined as χ v μ v 1. These charged ends can attract other water molecules. In this way a water molecule is somewhat like a magnet. The result is that while the molecule is electrically neutral it has poles just as magnets do. Just like a magnet water molecules attract each other due to their oppositely charged atoms.
 Source: evolving-innovation.com
Source: evolving-innovation.com
It has partial positive charge on the hydrogen atoms and the partial negative charge on the oxygen atom. Accordingly how is a water molecule like a magnet quizlet. Intermolecular forces can be of three different types. Just like a magnet water molecules attract each other due to their oppositely charged atoms. See full answer below.
Diamagnetic materials like water or water based materials have a relative magnetic permeability that is less than or equal to 1 and therefore a magnetic susceptibility less than or equal to 0 since susceptibility is defined as χ v μ v 1. See full answer below. Like a magnet and the earth. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. Because of this attraction the water molecules act like tiny magnets and cling to each other.
 Source: slideplayer.com
Source: slideplayer.com
See full answer below. Just like a magnet water molecules attract each other due to their oppositely charged atoms. The result is that while the molecule is electrically neutral it has poles just as magnets do. Because of this attraction the water molecules act like tiny magnets and cling to each other. Because of the natural polarity water molecules attract one another and stick together forming hydrogen bonds.
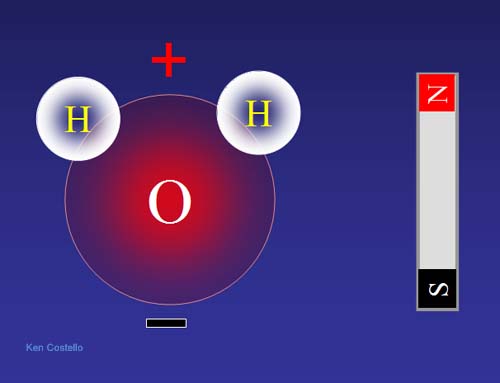 Source: chemistryland.com
Source: chemistryland.com
The hydrogen ions form a 104 5 degree angle with the oxygen molecule. The structure of the water molecule is a distorted tetrahedron. It has partial positive charge on the hydrogen atoms and the partial negative charge on the oxygen atom. A water molecule is a magnet because hydrogen and oxygen atoms have opposite charges therefore they attract nearby water molecules. Water is a polar molecule with a partially positive end and a partially negative end.
 Source: slideplayer.com
Source: slideplayer.com
The hydrogen ions form a 104 5 degree angle with the oxygen molecule. How is a water molecule like a magnet. Water h 2 o has 2 hydrogen atoms and 1 oxygen atom. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. In this way a water molecule is somewhat like a magnet.
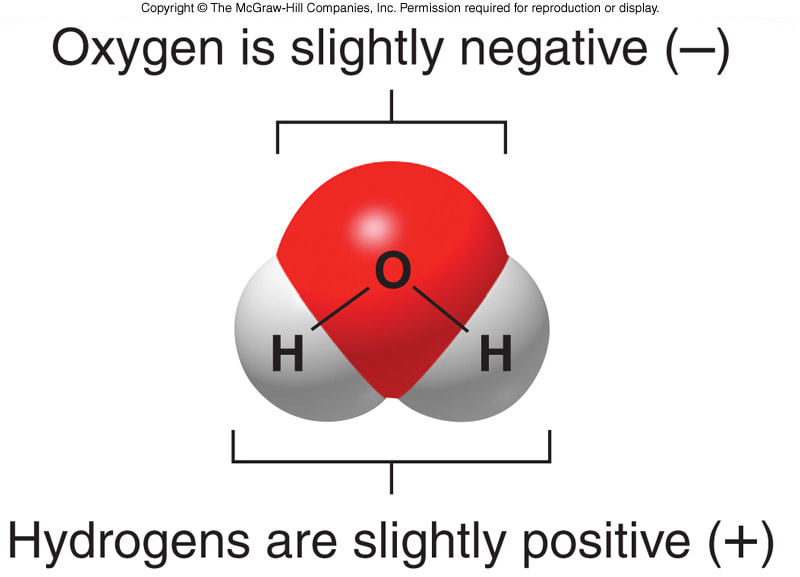 Source: taylorsciencegeeks.weebly.com
Source: taylorsciencegeeks.weebly.com
This attraction between the molecules is an intermolecular force force between different molecules. Explain how this takes place. See full answer below. Just like a magnet water molecules attract each other due to their oppositely charged atoms. Which property cohesion or adhesion causes surface tension in water.
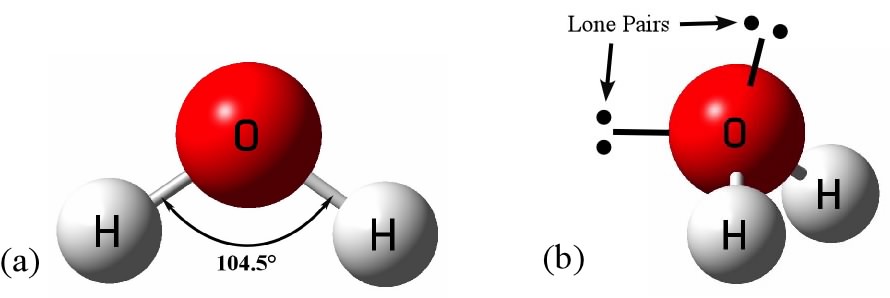 Source: socratic.org
Source: socratic.org
This forms very weak bonds between molecules called hydrogen bonds that are constantly being broken and reforming. Cohesion causes surface tension in water because of the high. Water h 2 o has 2 hydrogen atoms and 1 oxygen atom. The magnetic susceptibility values of various molecular fragments are called pascal s constants. These charged ends can attract other water molecules.
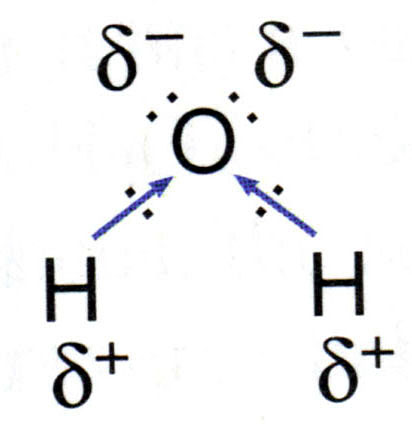 Source: socratic.org
Source: socratic.org
Accordingly how is a water molecule like a magnet quizlet. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. Explain how this takes place. Water is a polar molecule with a partially positive end and a partially negative end. These charged ends can attract other water molecules.
 Source: physicscentral.com
Source: physicscentral.com
Intermolecular forces can be of three different types. The negative side of of one molecule is attracted to the positive side of those around it. Water is a polar molecule which means that it has poles. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. Cohesion causes surface tension in water because of the high.
 Source: slideplayer.com
Source: slideplayer.com
Water h 2 o has 2 hydrogen atoms and 1 oxygen atom. The negative side of of one molecule is attracted to the positive side of those around it. See full answer below. The negative end of one water molecule attracts the positive ends of other water molecules surrounding it. Because of the natural polarity water molecules attract one another and stick together forming hydrogen bonds.

Intermolecular forces can be of three different types. The hydrogen ions form a 104 5 degree angle with the oxygen molecule. Additionally how is a water molecule polar. Because of the natural polarity water molecules attract one another and stick together forming hydrogen bonds. It has partial positive charge on the hydrogen atoms and the partial negative charge on the oxygen atom.
 Source: slideplayer.com
Source: slideplayer.com
It has partial positive charge on the hydrogen atoms and the partial negative charge on the oxygen atom. A water molecule is a magnet because hydrogen and oxygen atoms have opposite charges therefore they attract nearby water molecules. Because of this attraction the water molecules act like tiny magnets and cling to each other. This attraction between the molecules is an intermolecular force force between different molecules. The structure of the water molecule is a distorted tetrahedron.
 Source: study.com
Source: study.com
The negative end of one water molecule attracts the positive ends of other water molecules surrounding it. Just like a magnet water molecules attract each other due to their oppositely charged atoms. Water is a dipole and acts like a magnet with the oxygen end having a negative charge and the hydrogen end having a positive charge. Additionally how is a water molecule polar. Diamagnetic materials like water or water based materials have a relative magnetic permeability that is less than or equal to 1 and therefore a magnetic susceptibility less than or equal to 0 since susceptibility is defined as χ v μ v 1.
 Source: slideplayer.com
Source: slideplayer.com
The negative side of of one molecule is attracted to the positive side of those around it. Explain how this takes place. In this way a water molecule is somewhat like a magnet. Additionally how is a water molecule polar. The negative side of of one molecule is attracted to the positive side of those around it.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how is a water molecule like a magnet by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






