How electroplating is done
How Electroplating Is Done. High quality and safe nickel plating. This process is done through controlled depth plating which involves fixing the part in a manner that provides continuous electrical contact and submerges the area to be plated at defined depths through the plating solution. The electrolyte is a solution of a salt of the metal to be coated. Fully submerge your object in the electrolyte with one smooth motion.
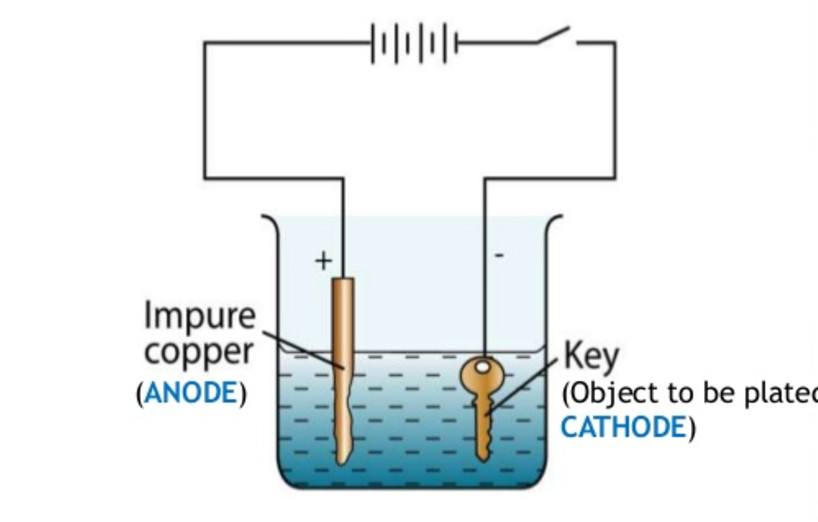 Electroplating Chemical Effect Of Electric Current Class 8 From classnotes.org.in
Electroplating Chemical Effect Of Electric Current Class 8 From classnotes.org.in
Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating. Electroplating is also known as electrodeposition. We will also be making our own electrolyte from scratch instead of buying chemicals online. This process is done through controlled depth plating which involves fixing the part in a manner that provides continuous electrical contact and submerges the area to be plated at defined depths through the plating solution. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. High quality and safe nickel plating.
A cell consists of two electrodes conductors usually made of metal which are held apart from one another.
Selective plating processes isolate the plating finish to a select area of the part. Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating. This process results in a thin layer of metal being deposited onto the surface of a workpiece called the substrate. Electroplating uses electrical currents to reduce dissolved metal cations. Electroplating involves passing an electric current through a solution called an electrolyte. How electroplating works.
 Source: quora.com
Source: quora.com
This partially dissolves the metals and creates a chemical bond between them. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Make sure the copper ball is attached to the positive terminal of the battery and that your object is connected to the negative terminal of your battery. Electroplating is the process of coating one metal or metal object with a very thin layer of another metal typically by applying a direct electric current. If this is done backwards the electroplating will not work and you will contaminate your electrolyte.
 Source: classnotes.org.in
Source: classnotes.org.in
The part to be coated acts as the cathode of an electrolytic cell. This process results in a thin layer of metal being deposited onto the surface of a workpiece called the substrate. We will also be making our own electrolyte from scratch instead of buying chemicals online. High quality and safe nickel plating. Electroplating is widely used in indus.
 Source: toppr.com
Source: toppr.com
Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating. Selective plating processes isolate the plating finish to a select area of the part. The electrolyte is a solution of a salt of the metal to be coated. High quality and safe nickel plating. Electroplating involves passing an electric current through a solution called an electrolyte.
 Source: feechiescience.wordpress.com
Source: feechiescience.wordpress.com
The electrodes and electrolyte are made from carefully chosen elements or compounds. How electroplating works. Electroplating is the process of coating one metal or metal object with a very thin layer of another metal typically by applying a direct electric current. The coating applied by electroplating is usually around 0 0002 inches thick. If you ve looked at my copper pl.
 Source: explainthatstuff.com
Source: explainthatstuff.com
The electrodes and electrolyte are made from carefully chosen elements or compounds. The current is provided by an external power supply. Electroplating is the process of coating one metal or metal object with a very thin layer of another metal typically by applying a direct electric current. Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating. The electrolyte is a solution of a salt of the metal to be coated.
 Source: thoughtco.com
Source: thoughtco.com
A cell consists of two electrodes conductors usually made of metal which are held apart from one another. If this is done backwards the electroplating will not work and you will contaminate your electrolyte. Selective plating processes isolate the plating finish to a select area of the part. Describing the plating of chrome copper nickel and gold. We will also be making our own electrolyte from scratch instead of buying chemicals online.
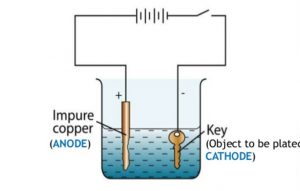 Source: classnotes.org.in
Source: classnotes.org.in
The current is provided by an external power supply. This is done to form a coherent metal coating on an electrode and change the surface properties of an object. This partially dissolves the metals and creates a chemical bond between them. The part to be coated acts as the cathode of an electrolytic cell. As the name suggests the process involves depositing material using an electric current.
 Source: en.wikipedia.org
Source: en.wikipedia.org
This partially dissolves the metals and creates a chemical bond between them. An explanation of the electroplating process. We will also be making our own electrolyte from scratch instead of buying chemicals online. Describing the plating of chrome copper nickel and gold. If you ve looked at my copper pl.
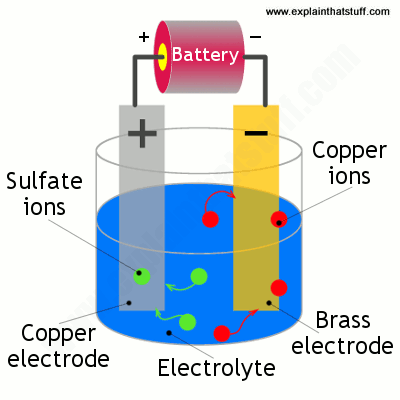 Source: explainthatstuff.com
Source: explainthatstuff.com
High quality and safe nickel plating. Electroplating is the application of electrolytic cells in which a thin layer of metal is deposited onto an electrically conductive surface. Describing the plating of chrome copper nickel and gold. The current is provided by an external power supply. Electroplating uses electrical currents to reduce dissolved metal cations.
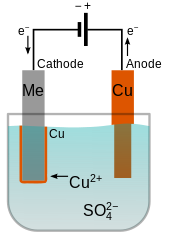 Source: en.wikipedia.org
Source: en.wikipedia.org
Selective plating processes isolate the plating finish to a select area of the part. The part to be coated acts as the cathode of an electrolytic cell. This process results in a thin layer of metal being deposited onto the surface of a workpiece called the substrate. This partially dissolves the metals and creates a chemical bond between them. Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating.
 Source: sciencestruck.com
Source: sciencestruck.com
A cell consists of two electrodes conductors usually made of metal which are held apart from one another. If this is done backwards the electroplating will not work and you will contaminate your electrolyte. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Describing the plating of chrome copper nickel and gold. This is done by dipping two terminals called electrodes into the electrolyte and connecting them into a circuit with a battery or other power supply.
 Source: yumpu.com
Source: yumpu.com
And the anode is usually either a block of that metal or of some inert conductive material. Electroplating is the process of coating one metal or metal object with a very thin layer of another metal typically by applying a direct electric current. Electroplating is primarily used to change the physical properties of an object. How electroplating works. The coating applied by electroplating is usually around 0 0002 inches thick.
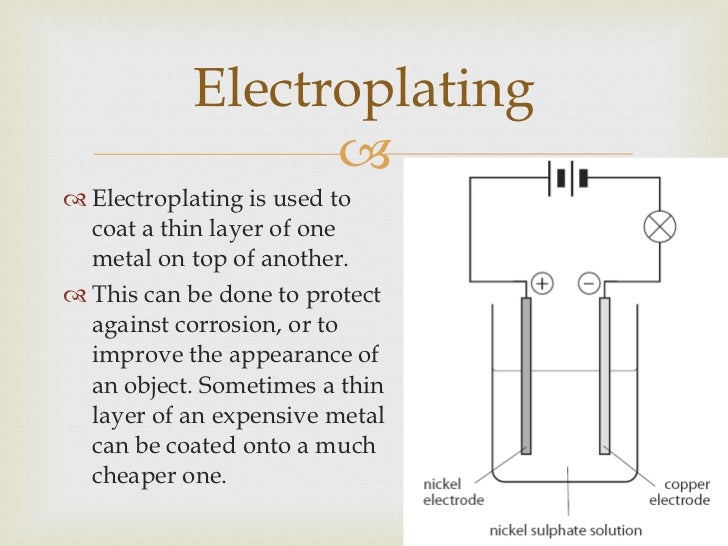 Source: slideshare.net
Source: slideshare.net
Electroplating uses electrical currents to reduce dissolved metal cations. Just like my popular copper plating instructable the aim of this is to do high quality low cost and safe electroplating. The electrodes are immersed in an electrolyte a solution. High quality and safe nickel plating. Make sure the copper ball is attached to the positive terminal of the battery and that your object is connected to the negative terminal of your battery.
 Source: britannica.com
Source: britannica.com
And the anode is usually either a block of that metal or of some inert conductive material. Electroplating involves passing an electric current through a solution called an electrolyte. This is done to form a coherent metal coating on an electrode and change the surface properties of an object. Selective plating processes isolate the plating finish to a select area of the part. Fully submerge your object in the electrolyte with one smooth motion.
 Source: youtube.com
Source: youtube.com
Describing the plating of chrome copper nickel and gold. A cell consists of two electrodes conductors usually made of metal which are held apart from one another. Selective plating processes isolate the plating finish to a select area of the part. The electrolyte is a solution of a salt of the metal to be coated. If you ve looked at my copper pl.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how electroplating is done by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






