How do acids and bases behave in water
How Do Acids And Bases Behave In Water. Here we will consider its ability to behave as an acid or a base. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water. Acids and bases play an important role in our lives. Pure hydrochloric acid is a gas but it dissolves easily in water to produce a solution of hydrogen ion and chloride ion.
 Acids And Bases Litmus Paper Properties Videos And Solved Questions From toppr.com
Acids And Bases Litmus Paper Properties Videos And Solved Questions From toppr.com
When dissolved in water acids donate hydrogen ions h. In some circumstances a water molecule will accept a proton and thus act as a brønsted lowry base. In each case the acid donates a proton to the base. Since nearly all of it is dissociated in water it is called a strong acid. Here we will consider its ability to behave as an acid or a base. Bases provide the oh ion.
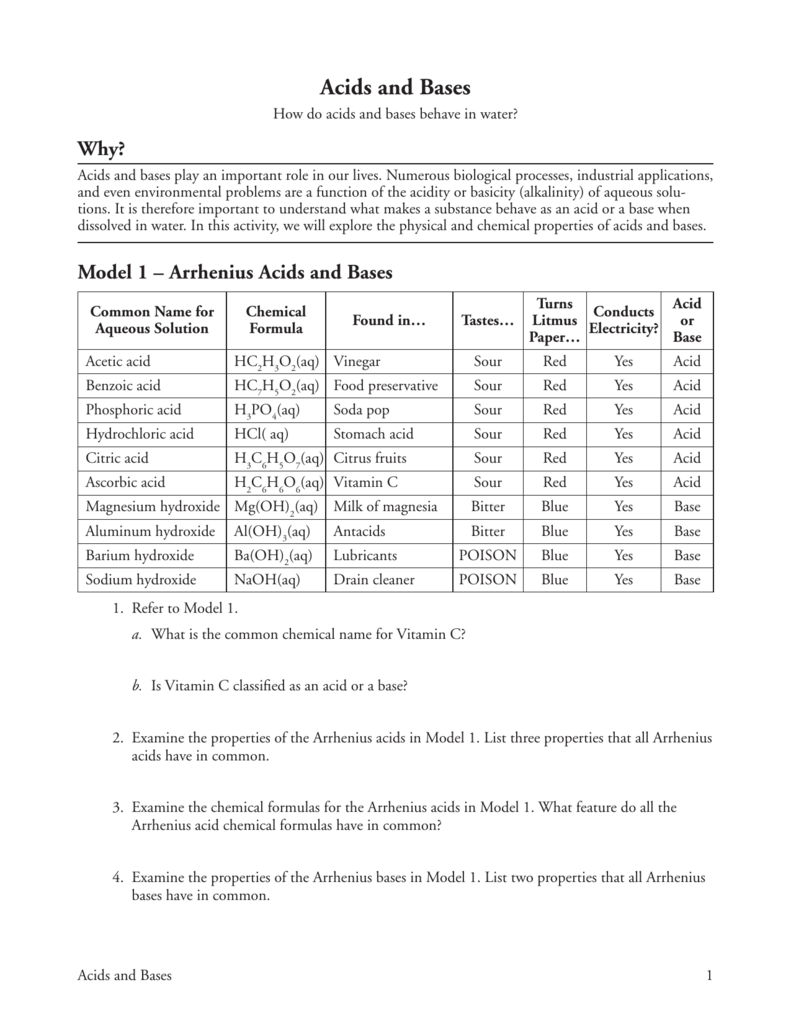
Numerous biological processes industrial applications and even environmental problems are a function of the acidity or basicity alkalinity of aqueous solu tions.
How do acids and bases behave in water. Since nearly all of it is dissociated in water it is called a strong acid. If a solution has a high concentration of h ions then it is acidic. Acids and base samaria etheridge how do acids and bases behave in water. To write chemical equations for water acting as an acid and as a base. Acids and bases play an important role in our lives.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Acids provide the h ion. Water h 2 o is an interesting compound in many respects. Acids that do not dissociate completely are called weak acids. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water. How do acids and bases behave in water.
 Source: butane.chem.uiuc.edu
Source: butane.chem.uiuc.edu
When dissolved in water acids donate hydrogen ions h. How do acids and bases behave in water. H aq oh aq h 2 o l the arrhenius theory has several disadvantages. To write chemical equations for water acting as an acid and as a base. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water.
Source:
To write chemical equations for water acting as an acid and as a base. Acids and base samaria etheridge how do acids and bases behave in water. Here we will consider its ability to behave as an acid or a base. Acids and bases play an important role in our lives. Pure hydrochloric acid is a gas but it dissolves easily in water to produce a solution of hydrogen ion and chloride ion.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
Numerous biological processes industrial applications and even environmental problems are a function of the acidity or basicity alkalinity of aqueous solutions. Numerous biological processes industrial applications and even environmental problems are a function of the acidity or basicity alkalinity of aqueous solutions. To write chemical equations for water acting as an acid and as a base. Lastly note that the reaction can proceed in either the forward or the backward direction. When dissolved in water acids donate hydrogen ions h.
 Source: studylib.net
Source: studylib.net
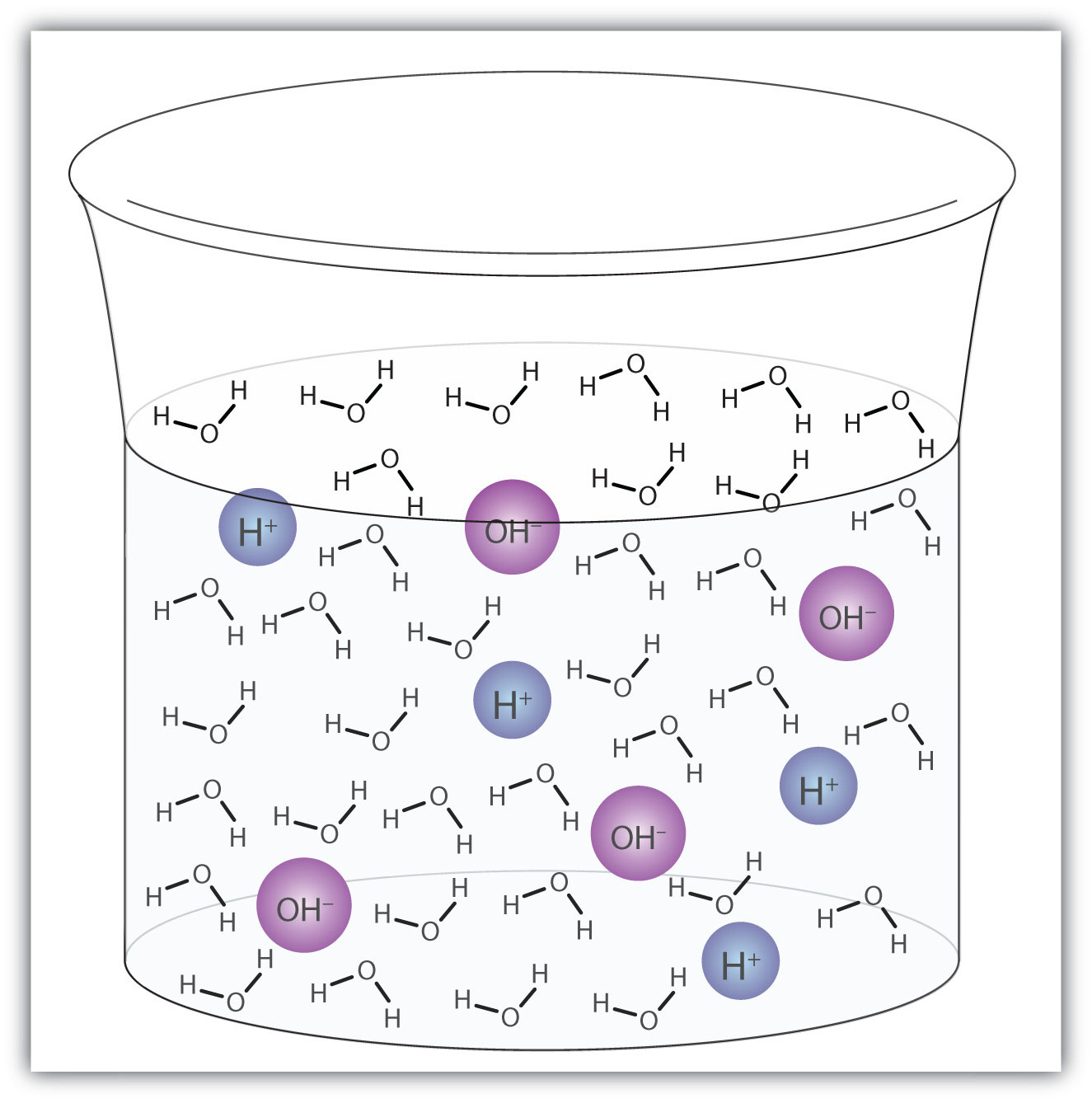
Acids and bases dissolve in water and because they increase the concentration of one of the products of water self ionization either protons or hydroxide ions they suppress water dissociation. Hydrogen ions are hydrogen atoms that have lost an electron and now have just a proton giving them a positive electrical charge. When dissolved in water acids donate hydrogen ions h. Acids and bases dissolve in water and because they increase the concentration of one of the products of water self ionization either protons or hydroxide ions they suppress water dissociation. Lastly note that the reaction can proceed in either the forward or the backward direction.
 Source: studylib.net
Source: studylib.net
Here we will consider its ability to behave as an acid or a base. For any acid k a is the equilibrium constant for the acid dissociation reaction in water. Acids that do not dissociate completely are called weak acids. It is therefore important to understand what makes a substance behave as an acid or a base when dissolved in water. In each case the acid donates a proton to the base.
 Source: slideplayer.com
Source: slideplayer.com
For any acid k a is the equilibrium constant for the acid dissociation reaction in water. For any acid k a is the equilibrium constant for the acid dissociation reaction in water. Water h 2 o is an interesting compound in many respects. It is therefore important to understand what makes a substance behave as an acid or a base when dissolved in water. Acids and bases play an important role in our lives.
 Source: toppr.com
Source: toppr.com
Since nearly all of it is dissociated in water it is called a strong acid. If a solution has a high concentration of h ions then it is acidic. Acids that do not dissociate completely are called weak acids. Acids provide the h ion. H aq oh aq h 2 o l the arrhenius theory has several disadvantages.
 Source: butane.chem.uiuc.edu
Source: butane.chem.uiuc.edu
Acids and base samaria etheridge how do acids and bases behave in water. When dissolved in water acids donate hydrogen ions h. Acids and bases play an important role in our lives. The strong acids turn into weak acids or i guess the acid does not react sense water is a base and bases are what deactivates the acids or clams them. Bases on the other hand mixed with water yield hydroxide ions oh.
 Source: bookpdfmanual.blogspot.com
Source: bookpdfmanual.blogspot.com
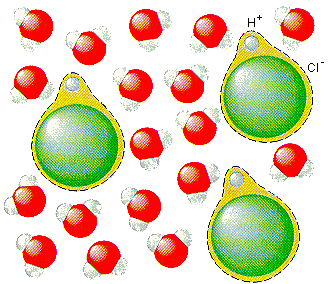
The acid illustrated is hydrochloric acid. For any acid k a is the equilibrium constant for the acid dissociation reaction in water. In each case the acid donates a proton to the base. In some circumstances a water molecule will accept a proton and thus act as a brønsted lowry base. To write chemical equations for water acting as an acid and as a base.
 Source: coursehero.com
Source: coursehero.com
If a solution has a high concentration of h ions then it is acidic. Acids that do not dissociate completely are called weak acids. Since nearly all of it is dissociated in water it is called a strong acid. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water. Lastly note that the reaction can proceed in either the forward or the backward direction.
Source:
How does water behave as how do acids and bases behave in water when they boric acid behaves as a lewis acid and accepts oh ions from water. Acids provide the h ion. And these ions combine to form water. In each case the acid donates a proton to the base. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water.
 Source: coursehero.com
Source: coursehero.com
Lastly note that the reaction can proceed in either the forward or the backward direction. Lastly note that the reaction can proceed in either the forward or the backward direction. How do acids and bases behave in water. Numerous biological processes industrial applications and even environmental problems are a function of the acidity or basicity alkalinity of aqueous solu tions. Here we will consider its ability to behave as an acid or a base.
 Source: slideplayer.com
Source: slideplayer.com
How does water behave as how do acids and bases behave in water when they boric acid behaves as a lewis acid and accepts oh ions from water. The acid illustrated is hydrochloric acid. In some circumstances a water molecule will accept a proton and thus act as a brønsted lowry base. Therefore according to the brønsted lowry definition an acid base reaction is one in which a conjugate base and a conjugate acid are formed note how this is different from the arrhenius definition of an acid base reaction which is limited to the reaction of h with oh to produce water. Acids and bases dissolve in water and because they increase the concentration of one of the products of water self ionization either protons or hydroxide ions they suppress water dissociation.
 Source: slideshare.net
Source: slideshare.net
Lastly note that the reaction can proceed in either the forward or the backward direction. Acids that do not dissociate completely are called weak acids. When dissolved in water acids donate hydrogen ions h. If a solution has a high concentration of h ions then it is acidic. Acids and bases play an important role in our lives.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how do acids and bases behave in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.