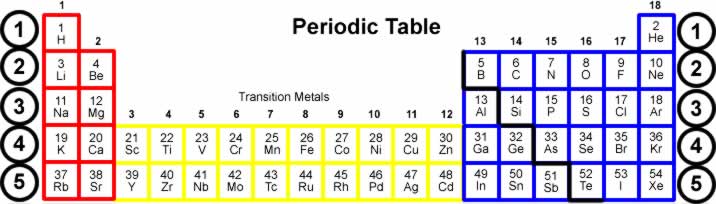
Home electrolysis of water
Home Electrolysis Of Water. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. This book comprehensively describes the fundamentals of electrochemical water electrolysis as well as the latest materials and technological developments. Electrolysis through several electrode plates and membranes the filtered water is separated into acidic and alkaline parts.
 Electrolysis Of Water Science Experiment The Joy Of Sharing From thejoysharing.com
Electrolysis Of Water Science Experiment The Joy Of Sharing From thejoysharing.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Split water into hydrogen and oxygen let me know if i should make this sort of video again have. It also includes an understanding of how materials and. The process of splitting water h 2 o into its atomic components hydrogen and oxygen using electricity is known as electrolysis. This electrolytic process is used in some industrial applications when hydrogen is needed. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications.
This process can be applied to fresh and salt water obtaining different results taking into account the product substances.
Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. Electrolysis through several electrode plates and membranes the filtered water is separated into acidic and alkaline parts. Remember high school chemistry lab. It addresses a variety of topics such as electrochemical processes materials components assembly and manufacturing and degradation mechanisms as well as challenges and strategies. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. The electrolysis of water allows the chemical elements that compose it to be obtained in a pure way that is hydrogen h through the cathode and oxygen o through the anode both in the gaseous state.
 Source: youtube.com
Source: youtube.com
It also includes an understanding of how materials and. The chemical change occurs when the substance loses electrons oxidation or gains them reduction. This book comprehensively describes the fundamentals of electrochemical water electrolysis as well as the latest materials and technological developments. In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. The process of splitting water h 2 o into its atomic components hydrogen and oxygen using electricity is known as electrolysis.
 Source: orbitingfrog.com
Source: orbitingfrog.com
Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. Split water into hydrogen and oxygen let me know if i should make this sort of video again have. This electrolytic process is used in some industrial applications when hydrogen is needed. It addresses a variety of topics such as electrochemical processes materials components assembly and manufacturing and degradation mechanisms as well as challenges and strategies.
 Source: m.youtube.com
Source: m.youtube.com
Electrolysis is responsible for a small percentage of water line breaks in nyc each year but it does play a role. This experiment has significant implications in terms of what these 2 gases can be used for in their own right with hydrogen being one of the cleanest sources of energy we have access to. You can do this at home. Electrolysis is responsible for a small percentage of water line breaks in nyc each year but it does play a role. This book comprehensively describes the fundamentals of electrochemical water electrolysis as well as the latest materials and technological developments.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. It also includes an understanding of how materials and. The electrolysis of water allows the chemical elements that compose it to be obtained in a pure way that is hydrogen h through the cathode and oxygen o through the anode both in the gaseous state. The chemical change occurs when the substance loses electrons oxidation or gains them reduction. Electrolysis of water electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water.
 Source: wikihow.com
Source: wikihow.com
This reaction takes place in a unit called an electrolyzer. You can do this at home. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Electrolysis through several electrode plates and membranes the filtered water is separated into acidic and alkaline parts. Electrolysis is the process of using electricity to split water into hydrogen and oxygen.
 Source: orbitingfrog.com
Source: orbitingfrog.com
It addresses a variety of topics such as electrochemical processes materials components assembly and manufacturing and degradation mechanisms as well as challenges and strategies. Split water into hydrogen and oxygen let me know if i should make this sort of video again have. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. Electrolysis is a promising option for green hydrogen production from renewable resources. This experiment has significant implications in terms of what these 2 gases can be used for in their own right with hydrogen being one of the cleanest sources of energy we have access to.
 Source: sciencenotes.org
Source: sciencenotes.org
Filtration the internal high grade filter filters chlorine unpleasant taste and odor and reduces sediments up to 5 micrometers to produce clean water. The electrolysis of water allows the chemical elements that compose it to be obtained in a pure way that is hydrogen h through the cathode and oxygen o through the anode both in the gaseous state. The process of splitting water h 2 o into its atomic components hydrogen and oxygen using electricity is known as electrolysis. This book comprehensively describes the fundamentals of electrochemical water electrolysis as well as the latest materials and technological developments. Remember high school chemistry lab.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Split water into hydrogen and oxygen let me know if i should make this sort of video again have. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. Remember high school chemistry lab. Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. Electrolysis of water electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water.
 Source: orbitingfrog.com
Source: orbitingfrog.com
In the two experiments listed below the first reactive substance is water and the second one is a copper sulfate solution. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. This experiment has significant implications in terms of what these 2 gases can be used for in their own right with hydrogen being one of the cleanest sources of energy we have access to. Electrolysis of water electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water. The chemical change occurs when the substance loses electrons oxidation or gains them reduction.
 Source: youtube.com
Source: youtube.com
Electrolysis is commercially highly important as a stage in the separation of elements from naturally occurring sources. The electrolysis of water allows the chemical elements that compose it to be obtained in a pure way that is hydrogen h through the cathode and oxygen o through the anode both in the gaseous state. Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. It also includes an understanding of how materials and. Electrolysis through several electrode plates and membranes the filtered water is separated into acidic and alkaline parts.
 Source: thejoysharing.com
Source: thejoysharing.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. This book comprehensively describes the fundamentals of electrochemical water electrolysis as well as the latest materials and technological developments. Electrolysis is commercially highly important as a stage in the separation of elements from naturally occurring sources. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas.
 Source: navigatingbyjoy.com
Source: navigatingbyjoy.com
You can do this at home. Electrolysis is responsible for a small percentage of water line breaks in nyc each year but it does play a role. Electrolysis is commercially highly important as a stage in the separation of elements from naturally occurring sources. Electrolysis is defined as a method of using a direct electric current dc to drive an otherwise non spontaneous chemical reaction. Remember high school chemistry lab.
 Source: youtube.com
Source: youtube.com
This process can be applied to fresh and salt water obtaining different results taking into account the product substances. You can do this at home. It addresses a variety of topics such as electrochemical processes materials components assembly and manufacturing and degradation mechanisms as well as challenges and strategies. It also includes an understanding of how materials and. The process of splitting water h 2 o into its atomic components hydrogen and oxygen using electricity is known as electrolysis.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electrolysis through several electrode plates and membranes the filtered water is separated into acidic and alkaline parts. Electrolysis is a promising option for green hydrogen production from renewable resources. This experiment has significant implications in terms of what these 2 gases can be used for in their own right with hydrogen being one of the cleanest sources of energy we have access to. Filtration the internal high grade filter filters chlorine unpleasant taste and odor and reduces sediments up to 5 micrometers to produce clean water. It also includes an understanding of how materials and.
 Source: thejoysharing.com
Source: thejoysharing.com
Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. Electrolysis is the process by which an electric current is passed through a substance to affect a chemical change. This process can be applied to fresh and salt water obtaining different results taking into account the product substances. The process of splitting water h 2 o into its atomic components hydrogen and oxygen using electricity is known as electrolysis. Filtration the internal high grade filter filters chlorine unpleasant taste and odor and reduces sediments up to 5 micrometers to produce clean water.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title home electrolysis of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.