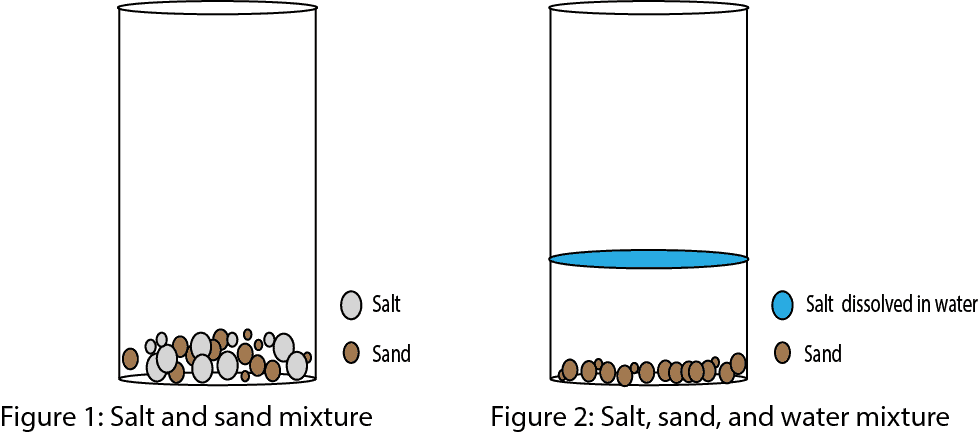
Fairly reactive metals
Fairly Reactive Metals. Not very reactive. Not very reactive lead. Fairly reactive carbon not a metal but included in series zinc. Hydrogen not a metal but included in series copper metal.
 Application Of The Reactivity Series Of Metals In The Extraction Of Metals A Plus Topper From aplustopper.com
Application Of The Reactivity Series Of Metals In The Extraction Of Metals A Plus Topper From aplustopper.com
Hydrogen not a metal but included in series copper metal. Therefore they are ready to lose that one electron in ionic bonding with other elements. Not reactive gold metal. Fairly reactive carbon not a metal but included in series zinc. Not very reactive lead. The reason for using cold water rather than hot water for the reactions of alkali metals is that alkali metals are already reactive enough at room temperature.
These metals have only one electron in their outer shell.
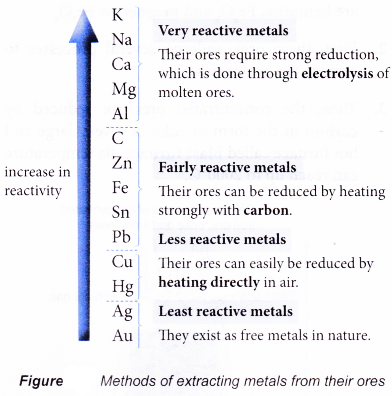
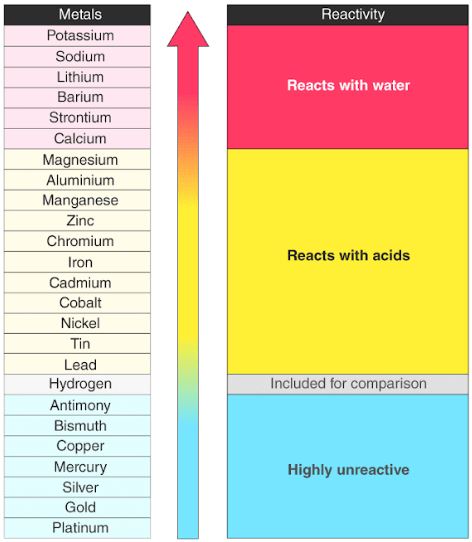
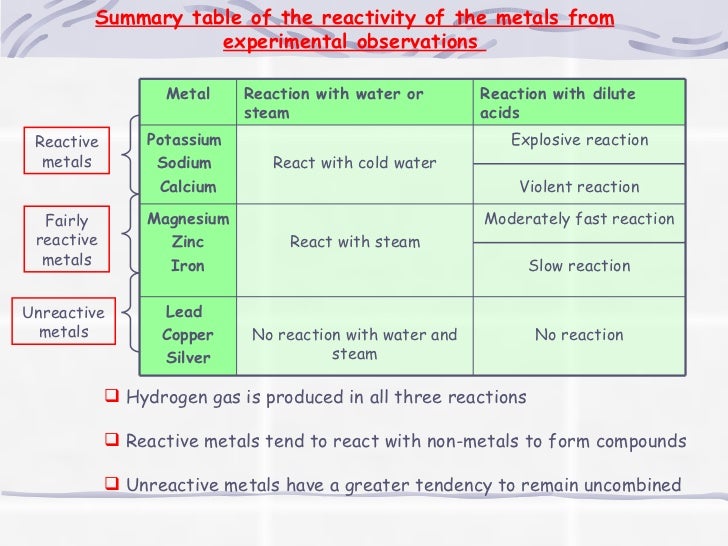
Not very reactive silver. Not very reactive silver. These metals have only one electron in their outer shell. Found as ores compounds often oxides or carbonates and can be extracted using carbon to reduce the metal compound to the pure metal. Even fairly small lumps of potassium will burn if you put them in cold water so imagine what it would be like with hot water. Copper is the least reactive of these five metals.
 Source: byjus.com
Source: byjus.com
These metals have only one electron in their outer shell. The alkali metals found in group 1 of the periodic table are highly reactive metals that do not occur freely in nature. Groups 5 to 12 would all count as fairly reactive metals but os ir pt au are usually found as metals rather than ores while ru rh pd ag hg and cu are sometimes found as metals. Therefore they are ready to lose that one electron in ionic bonding with other elements. Metal oxide carbon metal carbon dioxide.
 Source: fliphtml5.com
Source: fliphtml5.com
Not very reactive lead. Copper is the least reactive of these five metals. The 4 reactive metals have a high density and are softer and have lower melting and boiling points than transition metals. These metals have only one electron in their outer shell. The 4 reactive metals are also malleable ductile and good conductors of electricity and heat.
 Source: aplustopper.com
Source: aplustopper.com
Even fairly small lumps of potassium will burn if you put them in cold water so imagine what it would be like with hot water. Not reactive platinum. Found as ores compounds often oxides or carbonates and can be extracted using carbon to reduce the metal compound to the pure metal. Metal oxide carbon metal carbon dioxide. Hydrogen not a metal but included in series copper metal.
 Source: slideplayer.com
Source: slideplayer.com
Fairly reactive aluminium metal. Not very reactive lead. Fairly reactive aluminium metal. Therefore they are ready to lose that one electron in ionic bonding with other elements. Not very reactive silver.
 Source: compoundchem.com
Source: compoundchem.com
Not reactive gold metal. Copper is the least reactive of these five metals. Not reactive platinum. These metals have only one electron in their outer shell. This works for zinc iron tin lead and copper.
 Source: slideshare.net
Source: slideshare.net
Groups 5 to 12 would all count as fairly reactive metals but os ir pt au are usually found as metals rather than ores while ru rh pd ag hg and cu are sometimes found as metals. Metal oxide carbon metal carbon dioxide. Aluminium is the most abundant metal in earth s crust and the third most abundant element. The alkali metals found in group 1 of the periodic table are highly reactive metals that do not occur freely in nature. Fairly reactive iron metal.
 Source: slideshare.net
Source: slideshare.net
In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Fairly reactive iron metal. The alkali metals found in group 1 of the periodic table are highly reactive metals that do not occur freely in nature. Therefore they are ready to lose that one electron in ionic bonding with other elements.
 Source: slideplayer.com
Source: slideplayer.com
Not reactive gold metal. The 4 reactive metals have a high density and are softer and have lower melting and boiling points than transition metals. Hydrogen not a metal but included in series copper metal. Not reactive platinum. Fairly reactive iron metal.
 Source: slideplayer.com
Source: slideplayer.com
The 4 reactive metals have a high density and are softer and have lower melting and boiling points than transition metals. Fairly reactive iron metal. Not reactive gold metal. Fairly reactive aluminium metal. Copper is the least reactive of these five metals.
 Source: zephyrus.co.uk
Source: zephyrus.co.uk
The 4 reactive metals are also malleable ductile and good conductors of electricity and heat. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Not reactive gold metal. Fairly reactive aluminium metal. The 4 reactive metals have a high density and are softer and have lower melting and boiling points than transition metals.
 Source: quora.com
Source: quora.com
Therefore they are ready to lose that one electron in ionic bonding with other elements. This works for zinc iron tin lead and copper. The alkali metals found in group 1 of the periodic table are highly reactive metals that do not occur freely in nature. Metal oxide carbon metal carbon dioxide. Not very reactive silver.
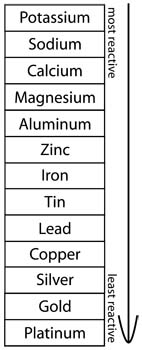 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
Groups 5 to 12 would all count as fairly reactive metals but os ir pt au are usually found as metals rather than ores while ru rh pd ag hg and cu are sometimes found as metals. Not very reactive. Groups 5 to 12 would all count as fairly reactive metals but os ir pt au are usually found as metals rather than ores while ru rh pd ag hg and cu are sometimes found as metals. The 4 reactive metals are also malleable ductile and good conductors of electricity and heat. Fairly reactive carbon not a metal but included in series zinc.
 Source: mammothmemory.net
Source: mammothmemory.net
Therefore they are ready to lose that one electron in ionic bonding with other elements. Fairly reactive carbon not a metal but included in series zinc. This works for zinc iron tin lead and copper. The alkali metals found in group 1 of the periodic table are highly reactive metals that do not occur freely in nature. Therefore they are ready to lose that one electron in ionic bonding with other elements.
 Source: quizlet.com
Source: quizlet.com
The 4 reactive metals are also malleable ductile and good conductors of electricity and heat. The 4 reactive metals are also malleable ductile and good conductors of electricity and heat. Copper is the least reactive of these five metals. Aluminium is the most abundant metal in earth s crust and the third most abundant element. Not reactive platinum.
 Source: slideshare.net
Source: slideshare.net
Fairly reactive iron metal. This works for zinc iron tin lead and copper. Fairly reactive aluminium metal. Found as ores compounds often oxides or carbonates and can be extracted using carbon to reduce the metal compound to the pure metal. Not reactive gold metal.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title fairly reactive metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.