Explain two ways in which water s properties help sustain life
Explain Two Ways In Which Water S Properties Help Sustain Life. Just as the negative end of a magnet will attract positive. Water s high specific heat helps animals by helping regulate their body temps as they sweat or pant. Water requires a loss of lot of heat to freeze. Water can moderate temperature because of the two properties.
 Why Does Water Support Life Overview Examples Expii From expii.com
Why Does Water Support Life Overview Examples Expii From expii.com
Water has lower density on freezing. Explain two ways in which water s properties help sustain life. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. The humble water molecule is made up of two hydrogen atoms bonded to an oxygen atom. Water requires a loss of lot of heat to freeze. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius.
Water has maximum density at 4 c.
Capillarity is used by plants to get water from the ground through their roots and leaves. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. Capillarity is used by plants to get water from the ground through their roots and leaves. Water has lower density on freezing. Explain two ways in which water s properties help sustain life. Water has high heat of fusion.
 Source: brainly.com
Source: brainly.com
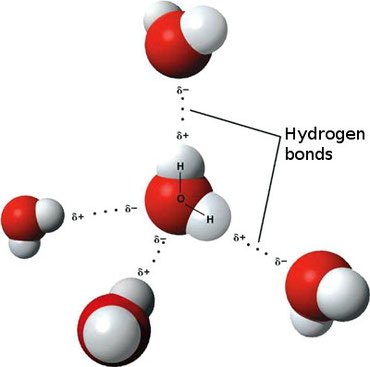
Because of these properties water store and spreads heat and prevent from overheating. Because of these properties water store and spreads heat and prevent from overheating. Water has maximum density at 4 c. Water has lower density on freezing. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet.
 Source: study.com
Source: study.com
Just as the negative end of a magnet will attract positive. Because of these properties water store and spreads heat and prevent from overheating. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. The way they re bonded together makes water this wonderful universal solvent meaning that almost every.
 Source: prezi.com
Source: prezi.com
Just as the negative end of a magnet will attract positive. Water has maximum density at 4 c. The way they re bonded together makes water this wonderful universal solvent meaning that almost every. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius. The humble water molecule is made up of two hydrogen atoms bonded to an oxygen atom.
 Source: withcarbon.com
Source: withcarbon.com
High specific heat and the high heat of vaporization. Because of these properties water store and spreads heat and prevent from overheating. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature. The humble water molecule is made up of two hydrogen atoms bonded to an oxygen atom. Ways water is stored.
 Source: expii.com
Source: expii.com
Capillarity and high specific heat. The way they re bonded together makes water this wonderful universal solvent meaning that almost every. Water has maximum density at 4 c. Explain two ways in which water s properties help sustain life. Water molecules form a lot of hydrogen bonds between one another.
 Source: expii.com
Source: expii.com
Ways water is stored. Water has maximum density at 4 c. Because of these properties water store and spreads heat and prevent from overheating. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius. Water can moderate temperature because of the two properties.
 Source: expii.com
Source: expii.com
Capillarity is used by plants to get water from the ground through their roots and leaves. Water can moderate temperature because of the two properties. Capillarity and high specific heat. The way they re bonded together makes water this wonderful universal solvent meaning that almost every. The humble water molecule is made up of two hydrogen atoms bonded to an oxygen atom.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
Water molecules form a lot of hydrogen bonds between one another. Water has lower density on freezing. Ways water is stored. Water molecules form a lot of hydrogen bonds between one another. High specific heat and the high heat of vaporization.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
Just as the negative end of a magnet will attract positive. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. Because of these properties water store and spreads heat and prevent from overheating. Water molecules form a lot of hydrogen bonds between one another. Just as the negative end of a magnet will attract positive.
 Source: expii.com
Source: expii.com
Water molecules form a lot of hydrogen bonds between one another. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature. Ways water is stored. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. Capillarity and high specific heat.
 Source: expii.com
Source: expii.com
Water s high specific heat helps animals by helping regulate their body temps as they sweat or pant. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature. Capillarity is used by plants to get water from the ground through their roots and leaves. Water molecules form a lot of hydrogen bonds between one another. Explain two ways in which water s properties help sustain life.
 Source: expii.com
Source: expii.com
Water can moderate temperature because of the two properties. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature. Capillarity and high specific heat. Ways water is stored. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius.
 Source: slideshare.net
Source: slideshare.net
Water has high heat of fusion. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius. Water requires a loss of lot of heat to freeze. Explain two ways in which water s properties help sustain life. The way they re bonded together makes water this wonderful universal solvent meaning that almost every.
 Source: expii.com
Source: expii.com
Because of these properties water store and spreads heat and prevent from overheating. The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. Water has high heat of fusion. The way they re bonded together makes water this wonderful universal solvent meaning that almost every. The humble water molecule is made up of two hydrogen atoms bonded to an oxygen atom.
 Source: sitn.hms.harvard.edu
Source: sitn.hms.harvard.edu
The hydrogen in the water is positively charged while the oxygen is negatively charged giving the molecule properties like a magnet. Water has lower density on freezing. Capillarity and high specific heat. High specific heat is the amount of energy that is absorbed or lost by one gram of a substance to change the temperature by 1 degree celsius. This prevents freezing and ice formation in the protoplasm even when exposed to very low temperature.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title explain two ways in which water s properties help sustain life by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.