Explain the physical properties of metals
Explain The Physical Properties Of Metals. Metals reflect equally nearly all visible electro magnetic waves. Metals have high tensile strength. Therefore the color of the most of the metals is white or silvery white except copper and gold. Form alloys with other metals.
 Physical Properties Of Metals And Nonmetals Toppr Guides From toppr.com
Physical Properties Of Metals And Nonmetals Toppr Guides From toppr.com
Metals are strong with high melting and boiling points. By considering the structure and bonding of metals which you have studied we can explain these properties. Heat can alter the mechanical properties of any metal like making a soft metal hard and then soft again. Metals can be hammered into thin sheets. Metals are a good conductor of heat and electricity. Form alloys with other metals.
Metals are a good conductor of heat and electricity.
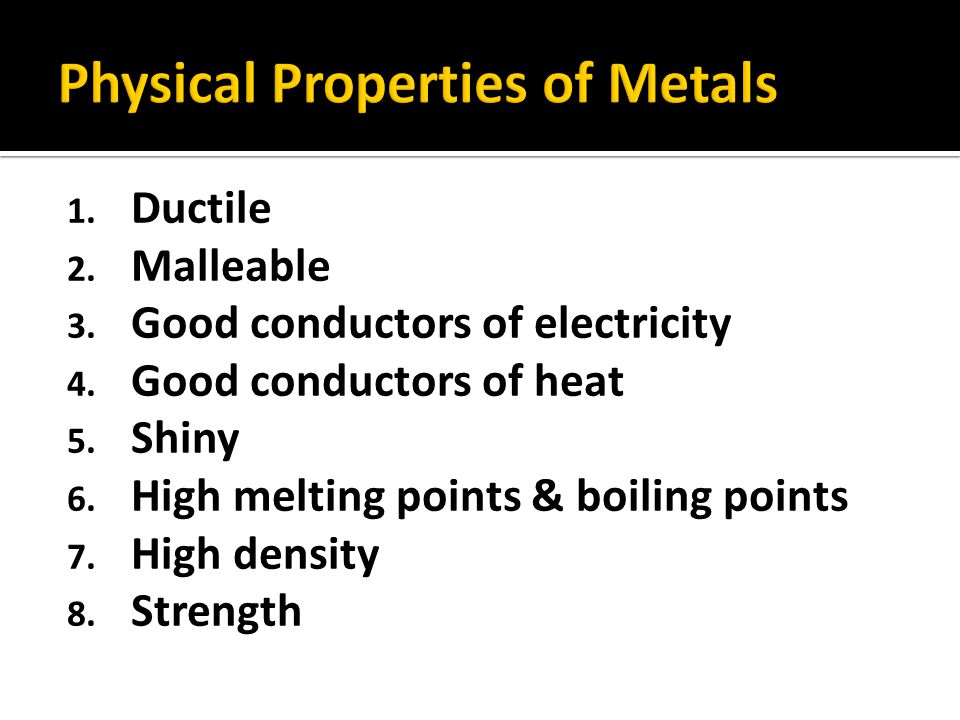
1 54 triple only explain typical physical properties of metals including electrical conductivity and malleability metals are good conductors because they have delocalised electrons which are free to move. They show following properties physical properties of metals. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience. Metals are lustrous which means they have a shiny appearance. Metalloids are metallic looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.
 Source: igcseaid.com
Source: igcseaid.com
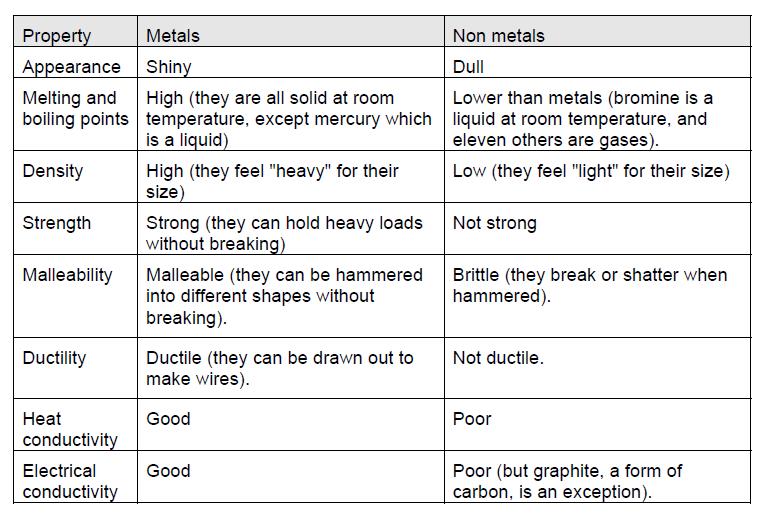
All metals are physically lustrous. All metals have a shiny appearance at least when freshly polished. Are good conductors of heat and electricity. Iron is used to make cars buildings ships etc. Form alloys with other metals.
 Source: wghsjuniorscience.weebly.com
Source: wghsjuniorscience.weebly.com
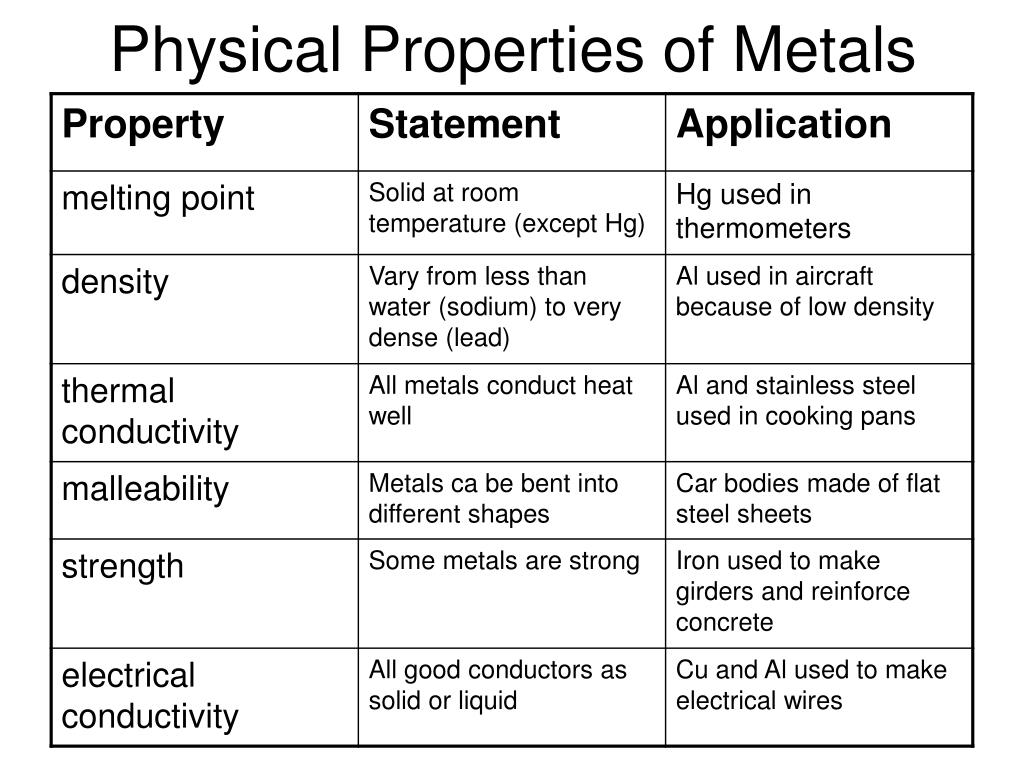
Gold is used for making jewellery. They show following properties physical properties of metals. Metals weigh a lot as they have a high density. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience. Metals are strong with high melting and boiling points.
 Source: slideplayer.com
Source: slideplayer.com
The delocalised electrons are free to move in the solid lattice. Metals weigh a lot as they have a high density. Fusing metals with compatible mechanical properties is an important welding skill because it can affect the product. They can be drawn into wires. All metals have a shiny appearance at least when freshly polished.
 Source: slideserve.com
Source: slideserve.com
Metals can be hammered into thin sheets. Therefore the color of the most of the metals is white or silvery white except copper and gold. Are good conductors of heat and electricity. They show following properties physical properties of metals. Physical properties of metals.
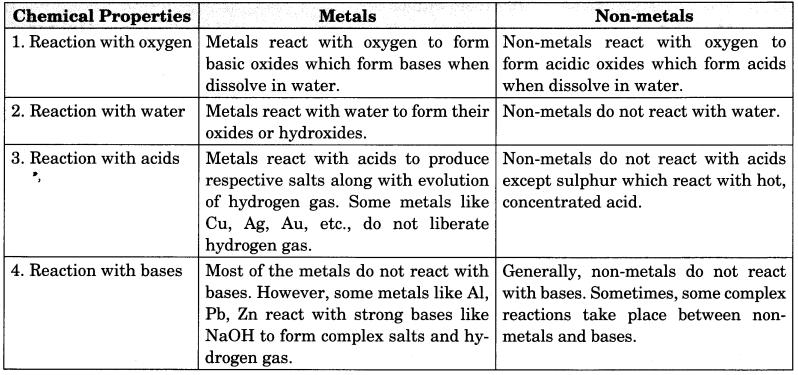 Source: learncbse.in
Source: learncbse.in
Metals are strong with high melting and boiling points. It means they possess the property of malleability. The measurable properties that allow a metal to resist external forces without failing are its mechanical properties. Iron is used to make cars buildings ships etc. They have a lustre that makes them shine.

Metals reflect equally nearly all visible electro magnetic waves. Therefore the color of the most of the metals is white or silvery white except copper and gold. Metals can be hammered into thin sheets. Metals are lustrous due to the. Gold is used for making jewellery.
 Source: slideplayer.com
Source: slideplayer.com
Metals can be hammered into thin sheets. Metals reflect equally nearly all visible electro magnetic waves. Metals are a good conductor of heat and electricity. Metals are strong with high melting and boiling points. They show following properties physical properties of metals.
 Source: toppr.com
Source: toppr.com
Metals are lustrous which means they have a shiny appearance. Form alloys with other metals. Metals are lustrous due to the. Iron is used to make cars buildings ships etc. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience.
 Source: brainly.in
Source: brainly.in
All metals are physically lustrous. Heat can alter the mechanical properties of any metal like making a soft metal hard and then soft again. By considering the structure and bonding of metals which you have studied we can explain these properties. Conductivity of heat and electricity solid and liquid metals conduct heat and electricity. Metals are a good conductor of heat and electricity.
 Source: brainly.in
Source: brainly.in
Iron is used to make cars buildings ships etc. Metals are hard they can t be broken easily and require a lot of energy and strength to break. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience. Conductivity of heat and electricity solid and liquid metals conduct heat and electricity. And have at least one basic oxide.
 Source: pinterest.com
Source: pinterest.com
By considering the structure and bonding of metals which you have studied we can explain these properties. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience. Metals are lustrous which means they have a shiny appearance. And have at least one basic oxide. Metals weigh a lot as they have a high density.
 Source: brainly.in
Source: brainly.in
Form alloys with other metals. Are good conductors of heat and electricity. Metals have a number of physical properties which make them useful to us in society and which you will be familiar with from everyday experience. Gold is used for making jewellery. It means they possess the property of malleability.
 Source: thoughtco.com
Source: thoughtco.com
Metals weigh a lot as they have a high density. Fusing metals with compatible mechanical properties is an important welding skill because it can affect the product. 1 54 triple only explain typical physical properties of metals including electrical conductivity and malleability metals are good conductors because they have delocalised electrons which are free to move. Form alloys with other metals. These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.
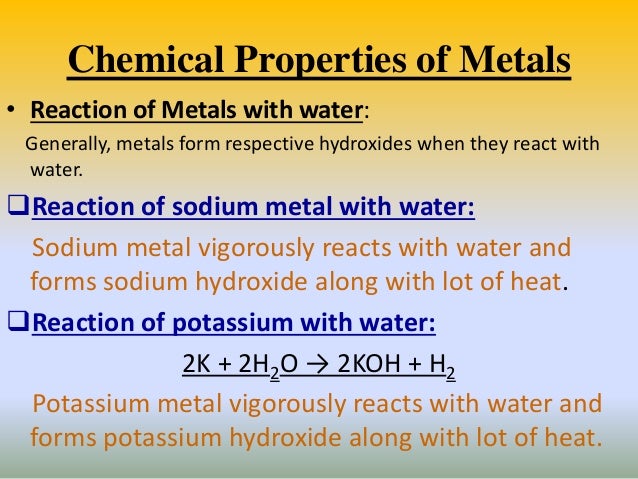 Source: slideshare.net
Source: slideshare.net
Metals are lustrous due to the. Metals are hard they can t be broken easily and require a lot of energy and strength to break. Metals are lustrous which means they have a shiny appearance. Metals can be hammered into thin sheets. These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.
 Source: thoughtco.com
Source: thoughtco.com
Metals are strong with high melting and boiling points. Heat can alter the mechanical properties of any metal like making a soft metal hard and then soft again. All metals have a shiny appearance at least when freshly polished. By considering the structure and bonding of metals which you have studied we can explain these properties. Metalloids are metallic looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title explain the physical properties of metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.