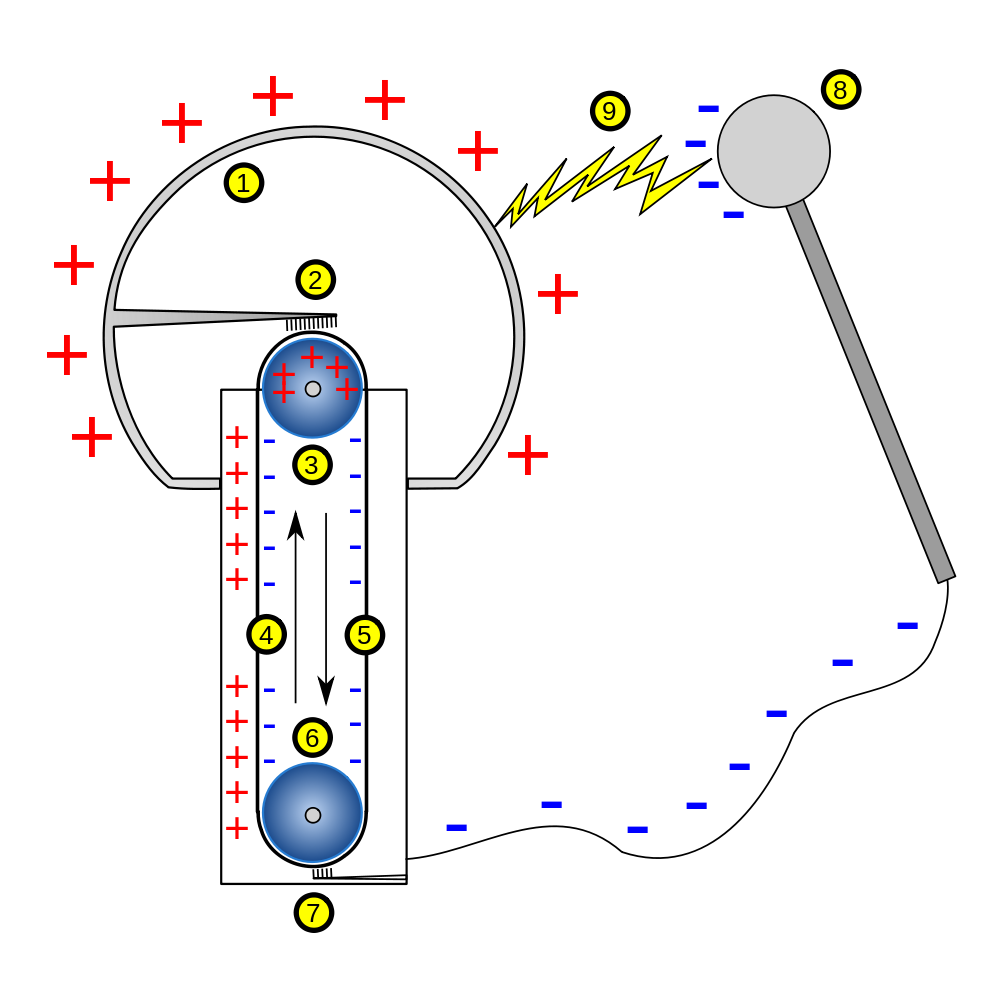
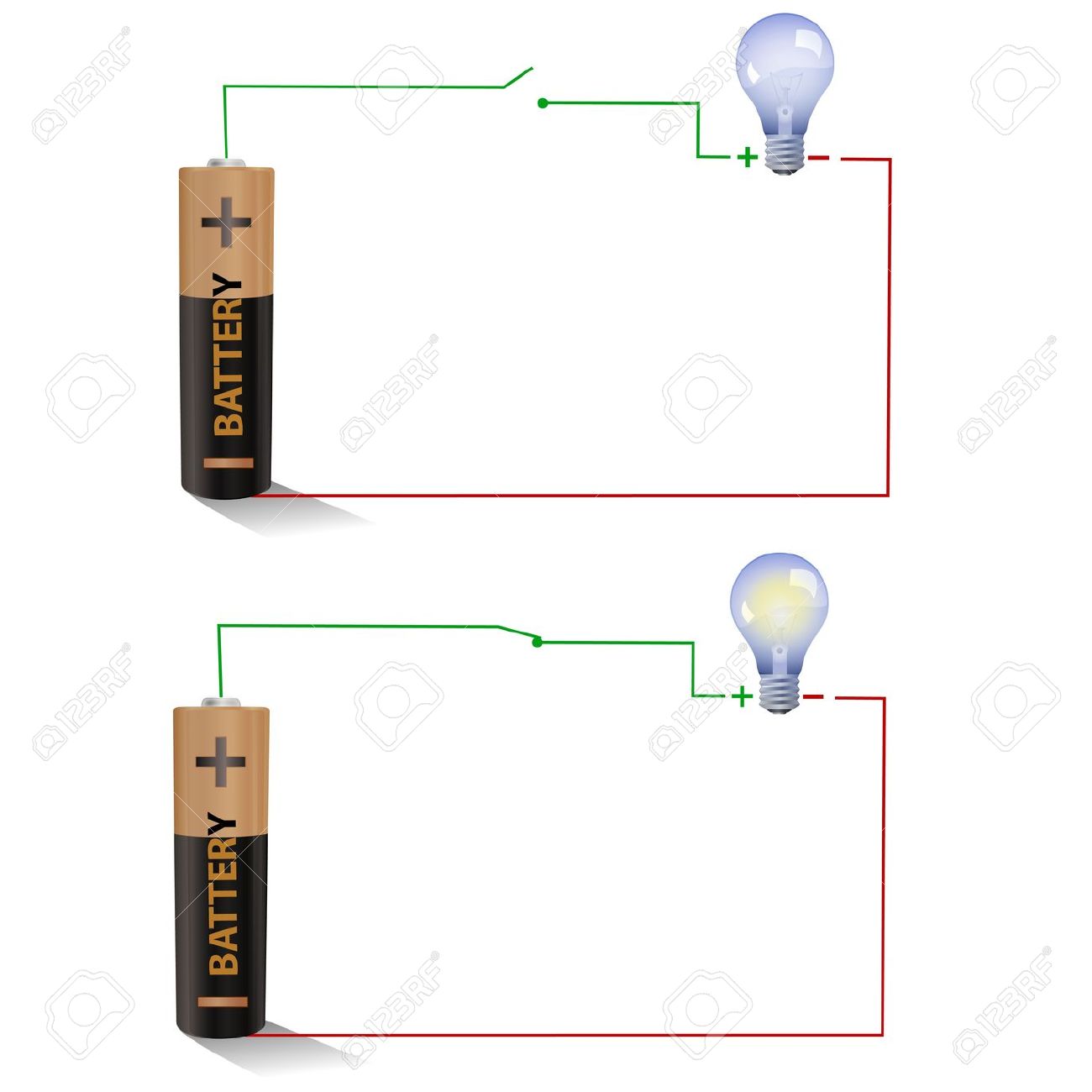
Experiment of electrolysis of water
Experiment Of Electrolysis Of Water. Water electrolysis splitting experiment hypothesis. So it cause metal corrosion and can be serious damage when it is exposed to human body. Try adding an electrolyte to the water in the beaker. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars.
 Pin On School From pinterest.com
Pin On School From pinterest.com
Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity. When this is done you should see a change in how quickly the bubbles form. You can get more information about how this experiment works in google. Water electrolysis is one of the most basic methods for producing hydrogen. 6 or 9 volt battery. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars.
All batteries have two terminals or ends.
You can get more information about how this experiment works in google. Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity. Piece of thin cardstock or cardboard. Small bubbles should start collecting around the pencil tips as electricity separates the water into two gases hydrogen and chlorine. Electrolysis of water experiment to break these bonds some amount of energy is required to get the atoms in water molecules active enough to break apart from each other. Water electrolysis is one of the most basic methods for producing hydrogen.

A positive terminal and a negative terminal. All batteries have two terminals or ends. Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below. Water electrolysis is one of the most basic methods for producing hydrogen. Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
6 or 9 volt battery. Water electrolysis splitting experiment hypothesis. When these bonds split apart the energy released can be used to do work. Hence experiments using cellulose. Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below.
 Source: kidslovekits.com
Source: kidslovekits.com
When these bonds split apart the energy released can be used to do work. So it cause metal corrosion and can be serious damage when it is exposed to human body. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars. Water electrolysis splitting experiment hypothesis. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes.
 Source: pinterest.com
Source: pinterest.com
Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below. Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity. Piece of thin cardstock or cardboard. When these bonds split apart the energy released can be used to do work. Electrolysis of water experiment energy is stored in the bonds of molecules.
 Source: pinterest.com
Source: pinterest.com
Water doesn t conduct electricity that well by itself but any electrolysis of water experiment could be accelerated by adding table salt to the water. So it cause metal corrosion and can be serious damage when it is exposed to human body. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars. Try adding an electrolyte to the water in the beaker. Water doesn t conduct electricity that well by itself but any electrolysis of water experiment could be accelerated by adding table salt to the water.
 Source: maddi603.wordpress.com
Source: maddi603.wordpress.com
Piece of thin cardstock or cardboard. Two insulated wires or alligator clip leads. Electrolysis of water experiment to break these bonds some amount of energy is required to get the atoms in water molecules active enough to break apart from each other. Hence experiments using cellulose. When this is done you should see a change in how quickly the bubbles form.
 Source: m.youtube.com
Source: m.youtube.com
Water electrolysis splitting experiment hypothesis. You can get more information about how this experiment works in google. Small bubbles should start collecting around the pencil tips as electricity separates the water into two gases hydrogen and chlorine. When these bonds split apart the energy released can be used to do work. When this is done you should see a change in how quickly the bubbles form.
 Source: thejoysharing.com
Source: thejoysharing.com
Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below. Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity. Water electrolysis splitting experiment hypothesis. Small bubbles should start collecting around the pencil tips as electricity separates the water into two gases hydrogen and chlorine. Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars.
 Source: en.wikipedia.org
Source: en.wikipedia.org
6 or 9 volt battery. Koh and naoh which are currently used as electrolytes for water electrolysis have strong alkalinity. Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below. A positive terminal and a negative terminal. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes.
 Source: orbitingfrog.com
Source: orbitingfrog.com
On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes. Electrolysis of water experiment energy is stored in the bonds of molecules. 6 or 9 volt battery. Hence experiments using cellulose. Hi guys watch this amazing video electrolysis of water experiment h2o electrolysis electrolysis water electrolysis chemistry like it and comments below.
 Source: scienceprojectideas.org
Source: scienceprojectideas.org
Keep in mind that chlorine is dangerous to inhale so make. Two insulated wires or alligator clip leads. Try adding an electrolyte to the water in the beaker. So it cause metal corrosion and can be serious damage when it is exposed to human body. Keep in mind that chlorine is dangerous to inhale so make.

Two insulated wires or alligator clip leads. You can get more information about how this experiment works in google. At home we can supply this energy with a battery. Electrolysis of water experiment to break these bonds some amount of energy is required to get the atoms in water molecules active enough to break apart from each other. When this is done you should see a change in how quickly the bubbles form.
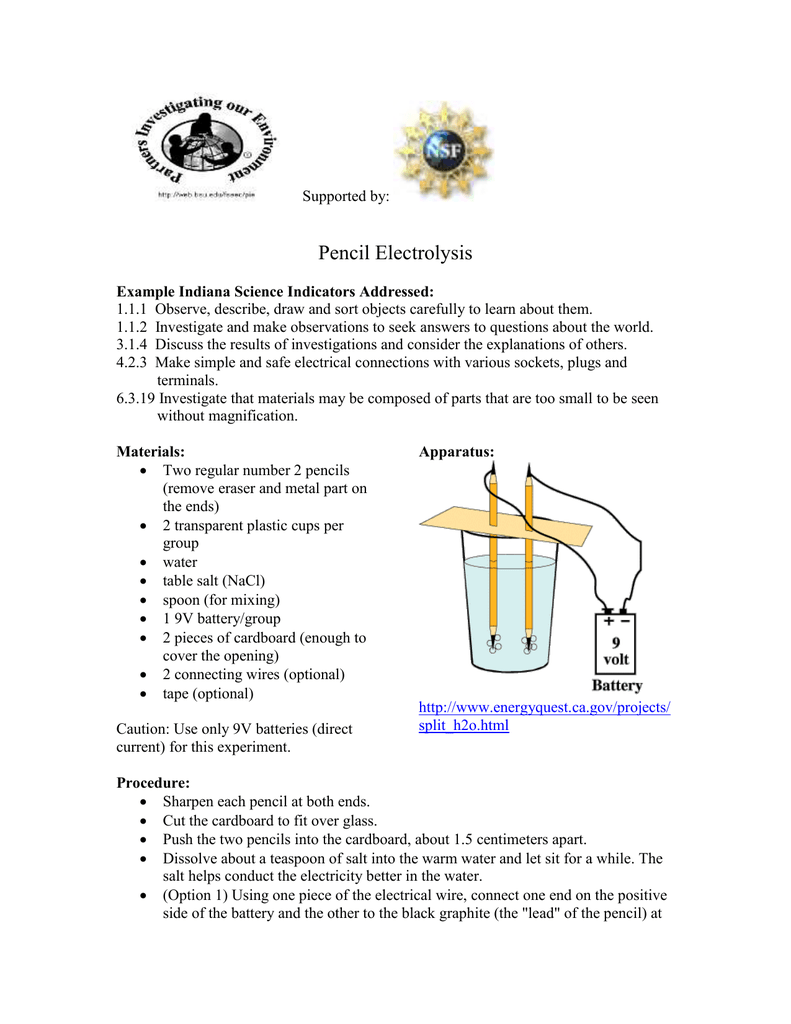 Source: studylib.net
Source: studylib.net
6 or 9 volt battery. Try adding an electrolyte to the water in the beaker. All batteries have two terminals or ends. You can get more information about how this experiment works in google. Piece of thin cardstock or cardboard.
 Source: id.pinterest.com
Source: id.pinterest.com
On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes. So it cause metal corrosion and can be serious damage when it is exposed to human body. Two insulated wires or alligator clip leads. All batteries have two terminals or ends. You can get more information about how this experiment works in google.
 Source: revision.co.zw
Source: revision.co.zw
Breaking apart liquid water molecules into hydrogen and oxygen gas creates an enormous amount of energy which can be turned into useful electricity to power our homes and cars. When this is done you should see a change in how quickly the bubbles form. Hence experiments using cellulose. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes. Small bubbles should start collecting around the pencil tips as electricity separates the water into two gases hydrogen and chlorine.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title experiment of electrolysis of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.