Elements compounds and mixtures examples
Elements Compounds And Mixtures Examples. A more technical definition of an alloy is a partial or complete solid solution of one or more elements in a metallic matrix. Shiny good conductors of thermal energy and electric current malleable can be hammered into thing sheets and ductile can be drawn into thin wires elements in this category iron tin lead copper. Mixtures are composed of variable proportions of molecules and atoms. A heterogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components can be visually distinguished and easily separated by physical means.
 Mixtures Elements Compounds Quiz Proprofs Quiz From proprofs.com
Mixtures Elements Compounds Quiz Proprofs Quiz From proprofs.com
Major categories of elements 1. While compounds and mixtures can be separated through a variety of techniques elements cannot as they exist in the purest form possible. Air is a mixture that contains the elements nitrogen oxygen and argon and also the compound carbon. Alloys mixtures in which the main elememt or elements are metal s. Shiny good conductors of thermal energy and electric current malleable can be hammered into thing sheets and ductile can be drawn into thin wires elements in this category iron tin lead copper. Colloids are very important in biology and medicine.
An example of an element is nitrogen atom n nitrogen gas n 2 nitrogen ion n 3 and nitrogen isotopes nitrogen 13 nitrogen 14 and nitrogen 15.
Mixtures are composed of variable proportions of molecules and atoms. Mixtures are composed of variable proportions of molecules and atoms. Mixtures can be a solid liquid or a gas. An example of an element is nitrogen atom n nitrogen gas n 2 nitrogen ion n 3 and nitrogen isotopes nitrogen 13 nitrogen 14 and nitrogen 15. Compounds are homogeneous forms of matter. There are many naturally occuring colloids e g.
 Source: proprofs.com
Source: proprofs.com
They are more easily separated than compounds. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together. Major categories of elements 1. Mixtures can usually be separated by physical techniques such as filtering. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here.
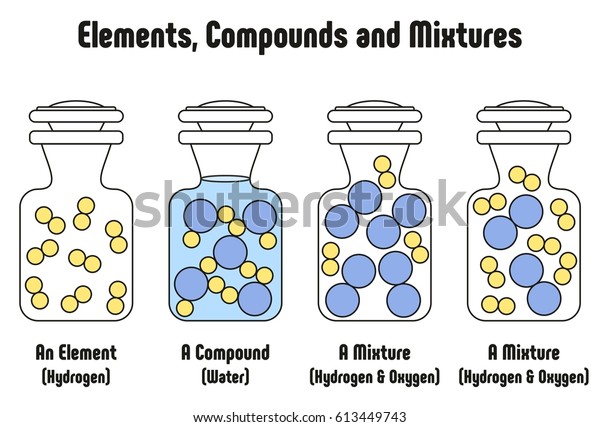 Source: shutterstock.com
Source: shutterstock.com
Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Mixtures are heterogeneous forms of matter. Air the cytosol of a cell. Elements exist in their simplest form and cannot be broken down further.
 Source: 123rf.com
Source: 123rf.com
There are many naturally occuring colloids e g. Examples of elements. Soil ocean water and other solutions. Compounds are homogeneous forms of matter. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here.
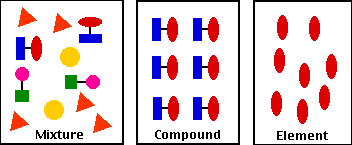 Source: biology-pages.info
Source: biology-pages.info
Shiny good conductors of thermal energy and electric current malleable can be hammered into thing sheets and ductile can be drawn into thin wires elements in this category iron tin lead copper. Mixtures can usually be separated by physical techniques such as filtering. Colloids are very important in biology and medicine. Water h2o salt nacl ammonia nh3 methane ch4 benzene c6h6. Examples of elements.
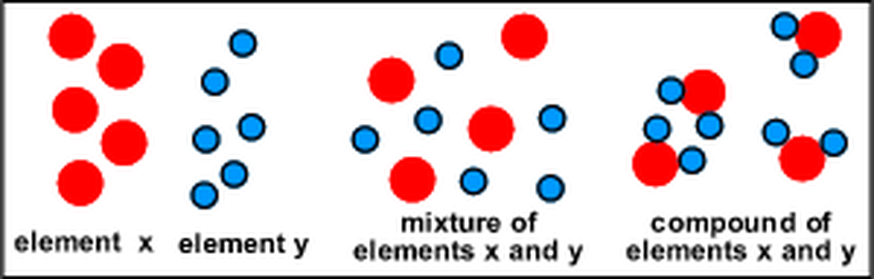 Source: sciencewitheberhart.weebly.com
Source: sciencewitheberhart.weebly.com
Air the cytosol of a cell. Major categories of elements 1. Mixtures can be a solid liquid or a gas. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. Elements exist in their simplest form and cannot be broken down further.
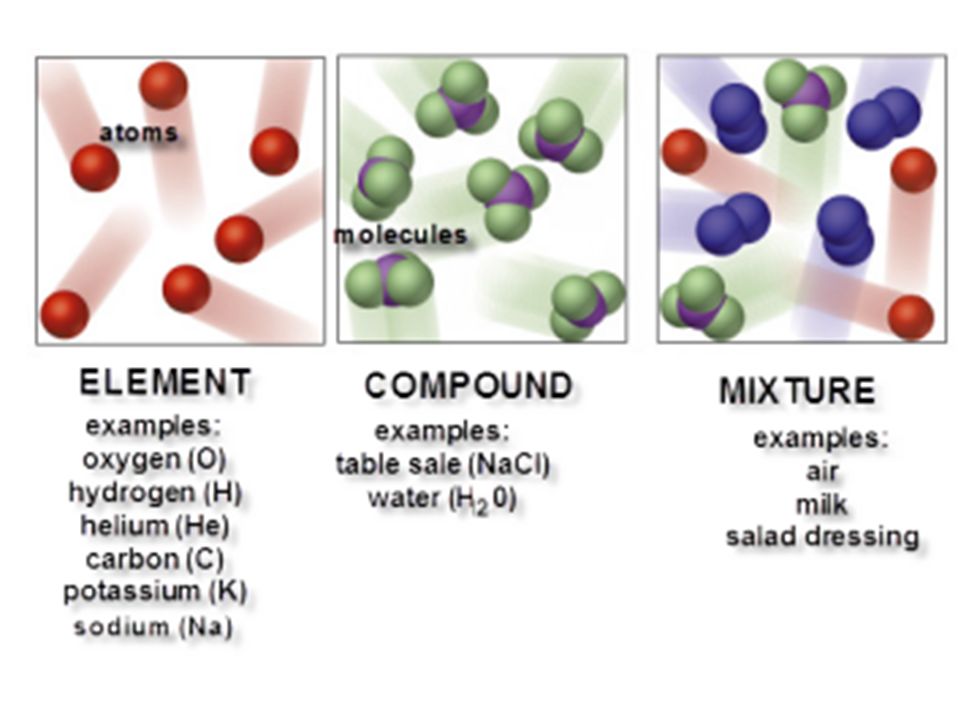
Air the cytosol of a cell. An example of an element is nitrogen atom n nitrogen gas n 2 nitrogen ion n 3 and nitrogen isotopes nitrogen 13 nitrogen 14 and nitrogen 15. While compounds and mixtures can be separated through a variety of techniques elements cannot as they exist in the purest form possible. Compounds are homogeneous forms of matter. A more technical definition of an alloy is a partial or complete solid solution of one or more elements in a metallic matrix.
 Source: sites.google.com
Source: sites.google.com
Soil ocean water and other solutions. Mixtures are heterogeneous forms of matter. Examples of elements. Elements exist in their simplest form and cannot be broken down further. Shiny good conductors of thermal energy and electric current malleable can be hammered into thing sheets and ductile can be drawn into thin wires elements in this category iron tin lead copper.
 Source: slideshare.net
Source: slideshare.net
Major categories of elements 1. A more technical definition of an alloy is a partial or complete solid solution of one or more elements in a metallic matrix. Mixtures can be a solid liquid or a gas. They are more easily separated than compounds. Soil ocean water and other solutions.
 Source: storyboardthat.com
Source: storyboardthat.com
Compounds are homogeneous forms of matter. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. So elements can exist in the form of ions atoms isotopes molecules. Mixtures can usually be separated by physical techniques such as filtering. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together.

Soil ocean water and other solutions. Colloids are very important in biology and medicine. Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. Mixtures are heterogeneous forms of matter. Mixtures are composed of variable proportions of molecules and atoms.
 Source: pinterest.com
Source: pinterest.com
Mixtures can be a solid liquid or a gas. Water h2o salt nacl ammonia nh3 methane ch4 benzene c6h6. So elements can exist in the form of ions atoms isotopes molecules. While compounds and mixtures can be separated through a variety of techniques elements cannot as they exist in the purest form possible. Alloys mixtures in which the main elememt or elements are metal s.
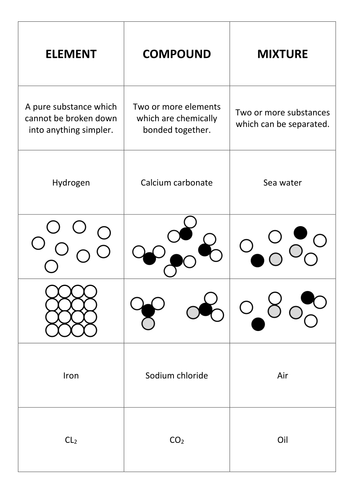
Examples of elements. Soil ocean water and other solutions. Water h2o salt nacl ammonia nh3 methane ch4 benzene c6h6. Examples of elements. Mixtures are heterogeneous forms of matter.
 Source: pinterest.com
Source: pinterest.com
Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. Their constituent elements atoms and or ions are always present in fixed proportions 1 1 depicted here. An example of an element is nitrogen atom n nitrogen gas n 2 nitrogen ion n 3 and nitrogen isotopes nitrogen 13 nitrogen 14 and nitrogen 15. Alloys mixtures in which the main elememt or elements are metal s. Water h2o salt nacl ammonia nh3 methane ch4 benzene c6h6.
 Source: examplesof.net
Source: examplesof.net
Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. Introducing students to atoms elements compounds and mixtures will provide them with an important foundation that will help them grasp more complex concepts in chemistry. There are many naturally occuring colloids e g. Elements exist in their simplest form and cannot be broken down further. Air the cytosol of a cell.
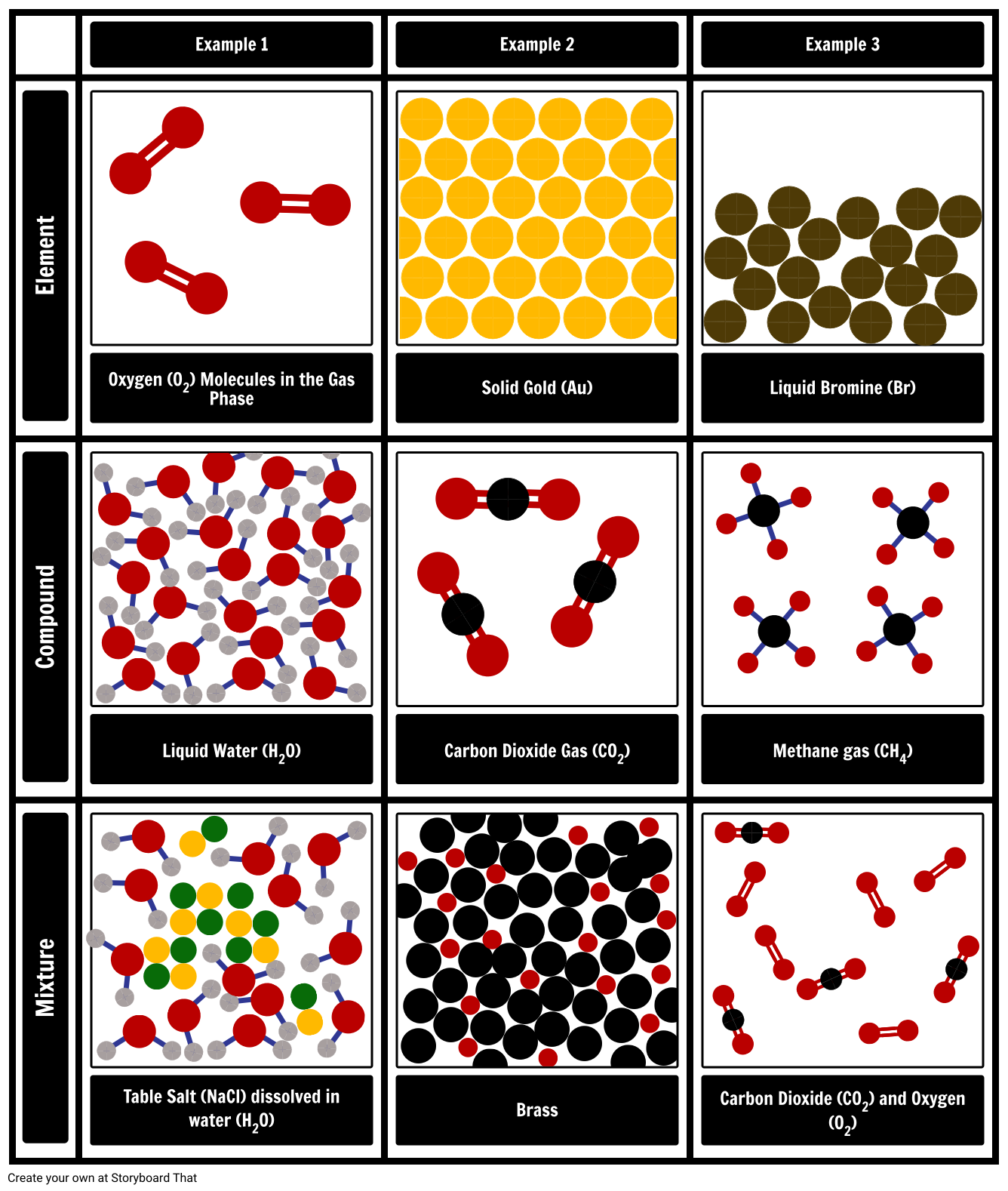 Source: storyboardthat.com
Source: storyboardthat.com
Mixtures are composed of variable proportions of molecules and atoms. An example of an element is nitrogen atom n nitrogen gas n 2 nitrogen ion n 3 and nitrogen isotopes nitrogen 13 nitrogen 14 and nitrogen 15. Water h2o salt nacl ammonia nh3 methane ch4 benzene c6h6. Mixtures can usually be separated by physical techniques such as filtering. Elements exist in their simplest form and cannot be broken down further.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title elements compounds and mixtures examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





