Element compound mixture examples
Element Compound Mixture Examples. You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen. Consist of two or more different elements and or compounds physically intermingled can be separated into their constituent parts by physical means e g. A heterogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components can be visually distinguished and easily separated by physical means. Mixtures are substances that are made of two or more types of element or compound that are not chemically bonded together.
 Section 1 Elements Compounds And Mixtures View As Single Page From open.edu
Section 1 Elements Compounds And Mixtures View As Single Page From open.edu
Brass is an example of a mixture of two elements. Example of compounds includes water h2o hydrogen peroxide h2o2 etc. Mixtures can be a solid liquid or a gas. Mixture of an element that exists in the form of molecules and a compound. It can contain as little as 10 or as much as 45 zinc. A compound is a substance that is made up of two or more elements whereas an element is a substance that cannot be split into simpler substances.
You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen.
In chemistry class we came to know the difference between a mixture compound and an element. Distilation of liquids or seperating magnetic and non magnetic solids using a magnet and. A heterogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components can be visually distinguished and easily separated by physical means. Water is made up of hydrogen and oxygen atoms. In chemistry class we came to know the difference between a mixture compound and an element. Consist of two or more different elements and or compounds physically intermingled can be separated into their constituent parts by physical means e g.
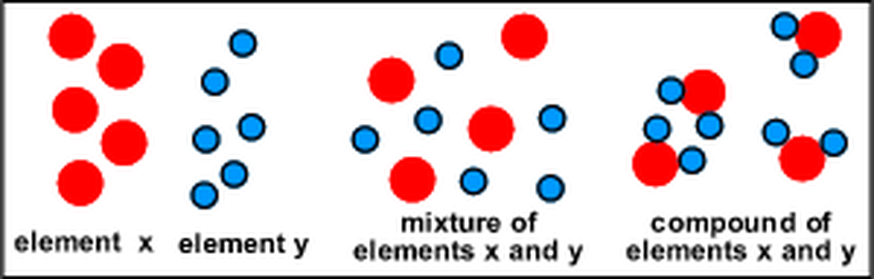 Source: sciencewitheberhart.weebly.com
Source: sciencewitheberhart.weebly.com
Distilation of liquids or seperating magnetic and non magnetic solids using a magnet and. Compound means a substance formed as a blend of various elements chemically in a certain proportion by weight. Distilation of liquids or seperating magnetic and non magnetic solids using a magnet and. Copper cu oxygen o hydrogen h helium he lithium li b compound. Examples of mixtures are sea water air and dirt.
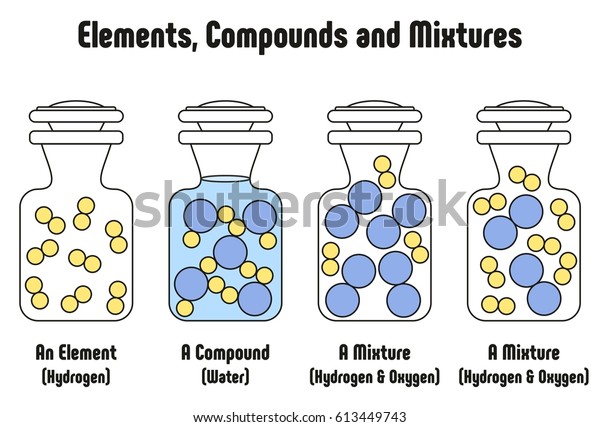 Source: shutterstock.com
Source: shutterstock.com
It can contain as little as 10 or as much as 45 zinc. Mixtures can be a solid liquid or a gas. Another difference between compounds and mixtures of elements is the ease with which the elements can be separated. Test out how much remember about the topic by taking up the test below. Examples of mixtures are sea water air and dirt.
 Source: slideshare.net
Source: slideshare.net
Consist of two or more different elements and or compounds physically intermingled can be separated into their constituent parts by physical means e g. Carbon dioxide is up of carbon and oxygen. A heterogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components can be visually distinguished and easily separated by physical means. Start studying element compound mixture examples. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4.
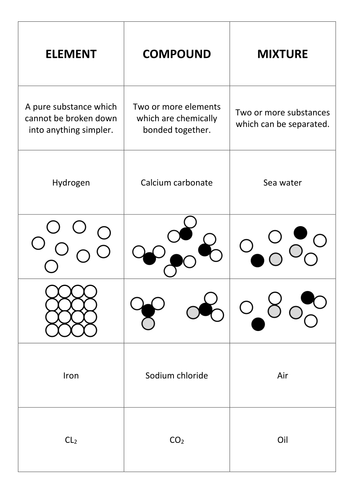
Mixtures such as the atmosphere contain two or more substances that are relatively easy to separate. In chemistry class we came to know the difference between a mixture compound and an element. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. A compound is a substance which contains two or more elements chemically combined together. Carbon dioxide is up of carbon and oxygen.
 Source: 123rf.com
Source: 123rf.com
Mixtures can be a solid liquid or a gas. Examples of different states in solutions gas in gas dry air oxygen in nitrogen gas in liquid soft drinks carbon dioxide in water liquid in liquid antifreeze alcohol in water solid in liquid salt water salt in water solid in solid brass zinc in copper. Brass is an example of a mixture of two elements. You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen. Start studying element compound mixture examples.
 Source: sites.google.com
Source: sites.google.com
Another difference between compounds and mixtures of elements is the ease with which the elements can be separated. You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen. Examples of different states in solutions gas in gas dry air oxygen in nitrogen gas in liquid soft drinks carbon dioxide in water liquid in liquid antifreeze alcohol in water solid in liquid salt water salt in water solid in solid brass zinc in copper. Mixtures can be a solid liquid or a gas. Water is made up of hydrogen and oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4. Mixture of an element that exists in the form of molecules and a compound. It is entirely new substance which possesses properties different from that of its constituent substances. A heterogeneous mixture is a mixture of two or more chemical substances elements or compounds where the different components can be visually distinguished and easily separated by physical means.
 Source: proprofs.com
Source: proprofs.com
Mixtures can be a solid liquid or a gas. Another difference between compounds and mixtures of elements is the ease with which the elements can be separated. For example water salt carbon dioxide sodium chloride etc. Examples of different states in solutions gas in gas dry air oxygen in nitrogen gas in liquid soft drinks carbon dioxide in water liquid in liquid antifreeze alcohol in water solid in liquid salt water salt in water solid in solid brass zinc in copper. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4.
 Source: examplesof.net
Source: examplesof.net
They are more easily separated than compounds. In chemistry class we came to know the difference between a mixture compound and an element. It is entirely new substance which possesses properties different from that of its constituent substances. Mixtures such as the atmosphere contain two or more substances that are relatively easy to separate. Learn vocabulary terms and more with flashcards games and other study tools.
 Source: storyboardthat.com
Source: storyboardthat.com
Distilation of liquids or seperating magnetic and non magnetic solids using a magnet and. A compound is a substance that is made up of two or more elements whereas an element is a substance that cannot be split into simpler substances. For example water salt carbon dioxide sodium chloride etc. Example of compounds includes water h2o hydrogen peroxide h2o2 etc. Brass is an example of a mixture of two elements.
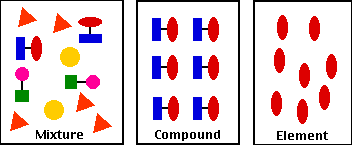 Source: biology-pages.info
Source: biology-pages.info
Brass is an example of a mixture of two elements. Water is made up of hydrogen and oxygen atoms. Compound means a substance formed as a blend of various elements chemically in a certain proportion by weight. Test out how much remember about the topic by taking up the test below. Examples of different states in solutions gas in gas dry air oxygen in nitrogen gas in liquid soft drinks carbon dioxide in water liquid in liquid antifreeze alcohol in water solid in liquid salt water salt in water solid in solid brass zinc in copper.
 Source: open.edu
Source: open.edu
Compound means a substance formed as a blend of various elements chemically in a certain proportion by weight. Example of compounds includes water h2o hydrogen peroxide h2o2 etc. For example water salt carbon dioxide sodium chloride etc. A compound is a substance that is made up of two or more elements whereas an element is a substance that cannot be split into simpler substances. Learn vocabulary terms and more with flashcards games and other study tools.
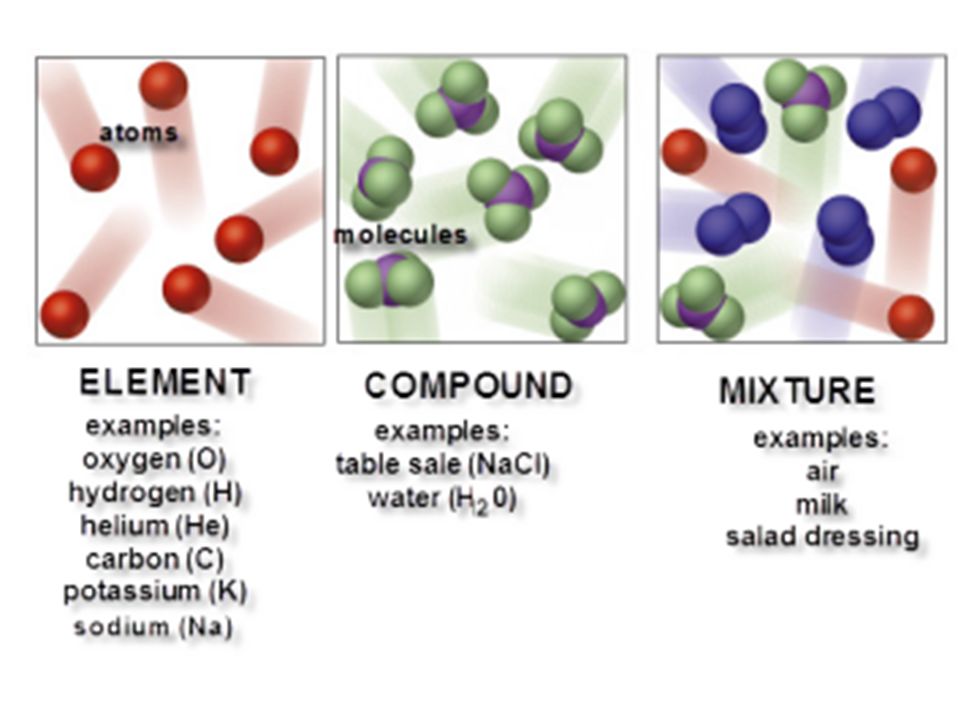
Consist of two or more different elements and or compounds physically intermingled can be separated into their constituent parts by physical means e g. Distilation of liquids or seperating magnetic and non magnetic solids using a magnet and. Water is made up of hydrogen and oxygen atoms. Mixture of an element that exists in the form of molecules and a compound. It can contain as little as 10 or as much as 45 zinc.
 Source: pinterest.com
Source: pinterest.com
Consist of two or more different elements and or compounds physically intermingled can be separated into their constituent parts by physical means e g. Start studying element compound mixture examples. You could see water s chemical formula it says it has 2 atoms of hydrogen combined with 1 atom of oxygen and in hydrogen peroxide it has 2 atoms of hydrogen and two atoms of oxygen. It can contain as little as 10 or as much as 45 zinc. For example water salt carbon dioxide sodium chloride etc.
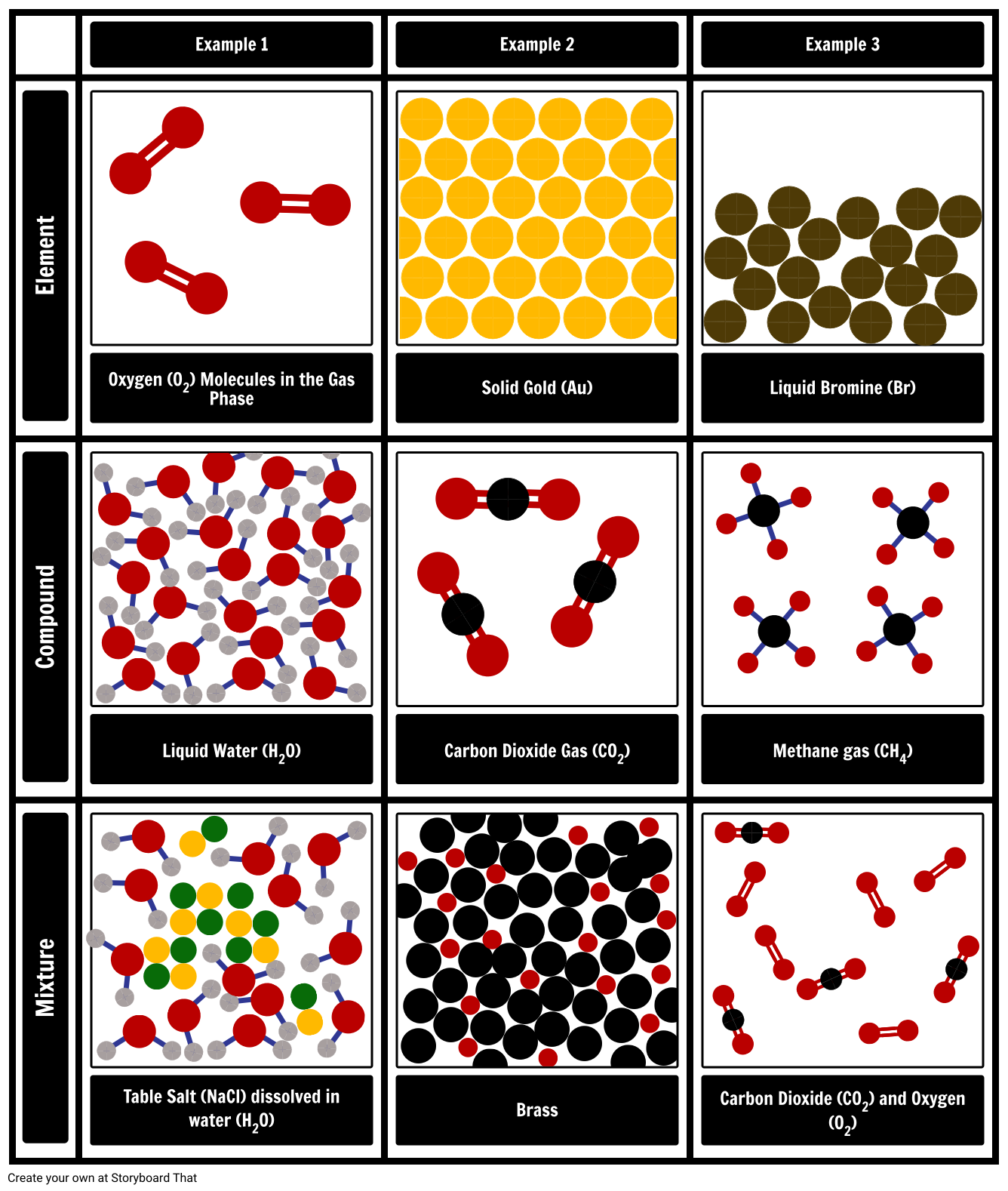 Source: storyboardthat.com
Source: storyboardthat.com
A compound is a substance which contains two or more elements chemically combined together. Water is made up of hydrogen and oxygen atoms. Example of compounds includes water h2o hydrogen peroxide h2o2 etc. Mixture of an element that exists in the form of molecules and a compound. Other examples of compounds include pure water h 2 o table salt nacl and methane ch 4.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title element compound mixture examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





