Electroplating of copper on iron
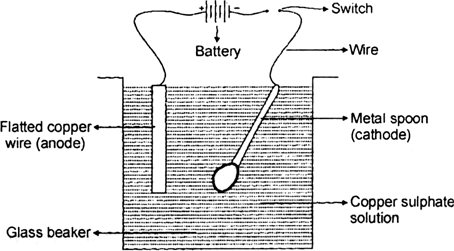
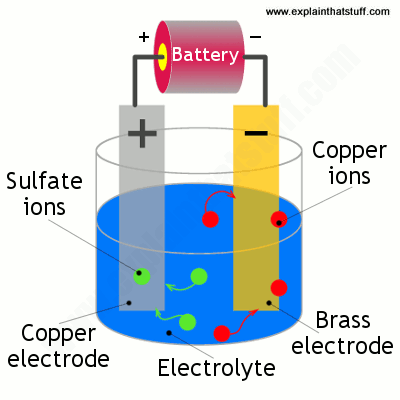
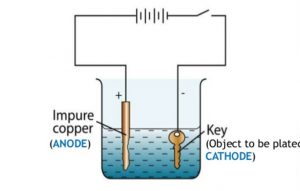
Electroplating Of Copper On Iron. 2 a copper plate is made positive electrode that is anode. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. Here the iron object on which electroplating takes place is made the cathode negative terminal. Your solution should be dark blue.
 Describe How You Electroplate An Iron Spoon With Copper 5 Scholr From scholr.com
Describe How You Electroplate An Iron Spoon With Copper 5 Scholr From scholr.com
Electroplating of iron onto copper soldering iron tips. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Preet s last name deleted for privacy by editor new delhi india. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. Your solution should be dark blue. This means that a copper plate is connected to the positive terminal of the battery.
A discussion started in 2002 but continuing through 2017.
This means that a copper plate is connected to the positive terminal of the battery. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Here the iron object on which electroplating takes place is made the cathode negative terminal. That s why we prefer to use zinc which is sacrificial to iron. To use an electrolytic cell to electroplate an object with copper items quantity 10 students 250 ml beakers 10 9v batteries 10 alligator clips 20 8 cm copper wire 20 pieces metal objects to plate iron strip 10 copper strips 10 distilled water 2l copper sulphate 50g electronic scale 1 ziploc bag 10. Preet s last name deleted for privacy by editor new delhi india.
 Source: zigya.com
Source: zigya.com
For electroplating an iron object with copper metal. For electroplating an iron object with copper metal. Electroplating of iron onto copper soldering iron tips. That s why we prefer to use zinc which is sacrificial to iron. To use an electrolytic cell to electroplate an object with copper items quantity 10 students 250 ml beakers 10 9v batteries 10 alligator clips 20 8 cm copper wire 20 pieces metal objects to plate iron strip 10 copper strips 10 distilled water 2l copper sulphate 50g electronic scale 1 ziploc bag 10.
 Source: explainthatstuff.com
Source: explainthatstuff.com
Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better. Your solution should be dark blue. Electrochemical series is the one in which metals are listed with other substances arranged in the order of. That s why we prefer to use zinc which is sacrificial to iron. How do we do iron coating on copper bits used in soldering irons.
 Source: youtube.com
Source: youtube.com
A discussion started in 2002 but continuing through 2017. Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better. A discussion started in 2002 but continuing through 2017. Following the copper strike. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied.
 Source: scholr.com
Source: scholr.com
This means that a copper plate is connected to the positive terminal of the battery. For electroplating an iron object with copper metal. Following the copper strike. 2 a copper plate is made positive electrode that is anode. The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal.
 Source: classnotes.org.in
Source: classnotes.org.in
To understand the process of electroplating let us take an example of electroplating iron objects with copper. Following the copper strike. Electrochemical series is the one in which metals are listed with other substances arranged in the order of. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. Dry it off on a paper towel.
 Source: m.youtube.com
Source: m.youtube.com
Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal. How do we do iron coating on copper bits used in soldering irons. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied.
 Source: quora.com
Source: quora.com
Electrochemical series is the one in which metals are listed with other substances arranged in the order of. Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating. Electrochemical series is the one in which metals are listed with other substances arranged in the order of. Dry it off on a paper towel. To use an electrolytic cell to electroplate an object with copper items quantity 10 students 250 ml beakers 10 9v batteries 10 alligator clips 20 8 cm copper wire 20 pieces metal objects to plate iron strip 10 copper strips 10 distilled water 2l copper sulphate 50g electronic scale 1 ziploc bag 10.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal. Electroplating of iron onto copper soldering iron tips. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating.
 Source: student-sc.blogspot.com
Source: student-sc.blogspot.com
Your solution should be dark blue. Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. Preet s last name deleted for privacy by editor new delhi india. Here the iron object on which electroplating takes place is made the cathode negative terminal.
 Source: shaalaa.com
Source: shaalaa.com
How do we do iron coating on copper bits used in soldering irons. Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating. The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal. How do we do iron coating on copper bits used in soldering irons. To understand the process of electroplating let us take an example of electroplating iron objects with copper.
Source:
Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. The copper strike plating step consists of applying a thin layer of copper in a copper cyanide solution to enhance the conductive properties of the base metal. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal.
 Source: revision.co.zw
Source: revision.co.zw
Iron is used for the electroplating of so copper because iron falls above copper in the electrochemical series whereas silver will fall off below the copper in electrochemical series that makes it not reliable for the electroplating purpose. The copper strike plating step consists of applying a thin layer of copper in a copper cyanide solution to enhance the conductive properties of the base metal. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. The copper metal which is going to be plated as a layer on the iron object is made the anode positive terminal. How do we do iron coating on copper bits used in soldering irons.
 Source: yenka.com
Source: yenka.com
Your solution should be dark blue. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. The copper strike plating step consists of applying a thin layer of copper in a copper cyanide solution to enhance the conductive properties of the base metal. This means that a copper plate is connected to the positive terminal of the battery. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid.
 Source: brainly.in
Source: brainly.in
Stir copper sulfate into some hot water in a beaker until no more will dissolve. Electrochemical series is the one in which metals are listed with other substances arranged in the order of. To use an electrolytic cell to electroplate an object with copper items quantity 10 students 250 ml beakers 10 9v batteries 10 alligator clips 20 8 cm copper wire 20 pieces metal objects to plate iron strip 10 copper strips 10 distilled water 2l copper sulphate 50g electronic scale 1 ziploc bag 10. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better.
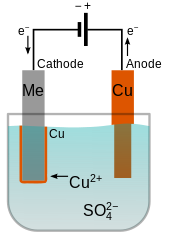 Source: en.wikipedia.org
Source: en.wikipedia.org
Electrochemical series is the one in which metals are listed with other substances arranged in the order of. Followed by strike plating of copper copper electroplating nickel electroplating and chromium electroplating. Dry it off on a paper towel. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electroplating of copper on iron by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.