Electroplating lab report
Electroplating Lab Report. Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects. Clean a small piece of the object to be plated with steel wool. The lab report is due friday evening. During electroplating a thin layer of a desirable metal is deposited onto another object.

Electroplating lab report 1297 words 6 pages title. To conclude our electroplating lab students will collaborate with their lab team to write a team lab report shared with the teacher as a google doc. A 3 4 inch long niobium wire was obtained and cleaned to remove fingerprints. Attach wire to the object. Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects. Current a an increase in the amount of current increases the amount of substance deposited in a fixed amount of time because there is more energy available to move ions electrons to the cathode from the anode.
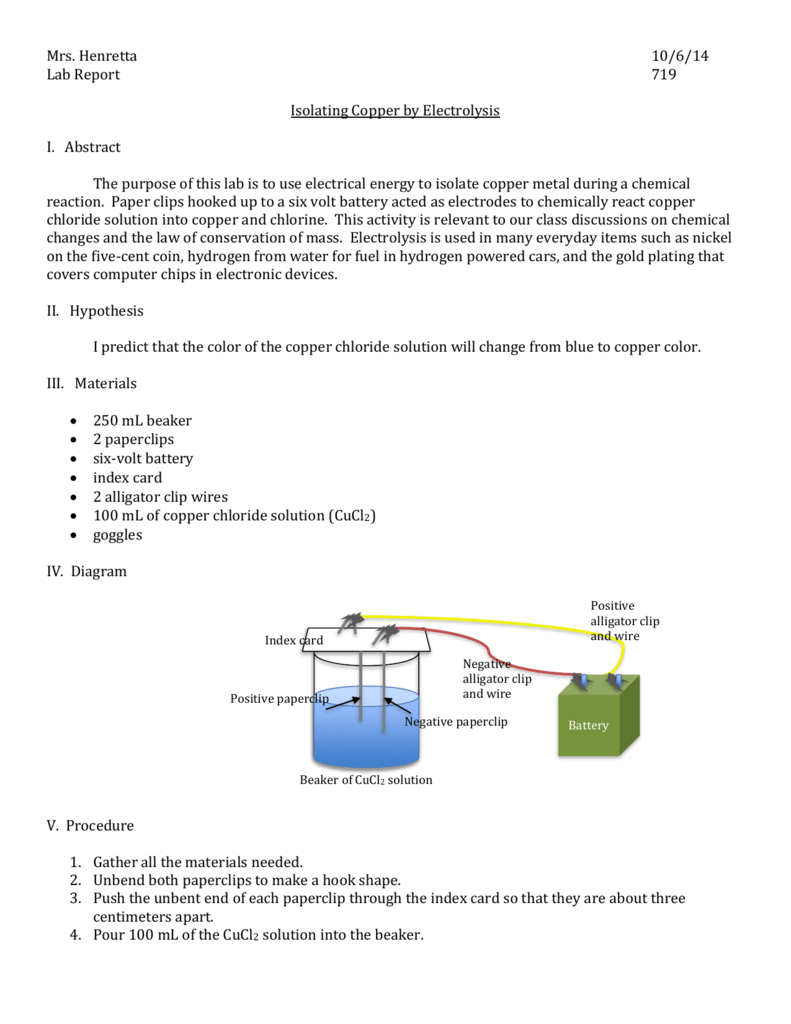
This lab is designed to provide an example of the reduction oxidation redox processes.
In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. During electroplating the object to be. The lab report is due friday evening. This lab is designed to provide an example of the reduction oxidation redox processes. Add 1 m cuso 4. Electroplating lab report 1297 words 6 pages title.
 Source: studylib.net
Source: studylib.net
In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. This lab is designed to provide an example of the reduction oxidation redox processes. Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects. During electroplating the object to be. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current.
 Source: studylib.net
Source: studylib.net
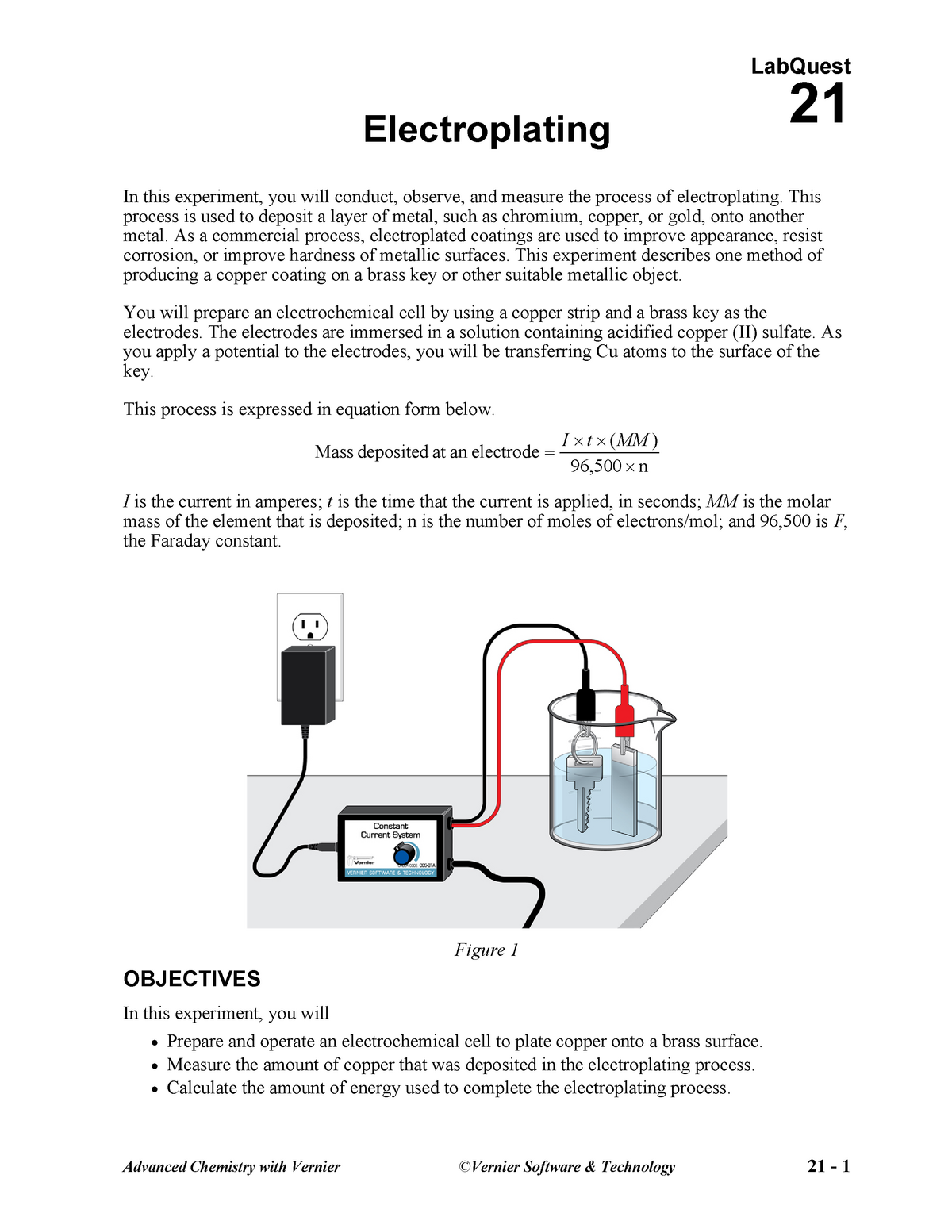
An investigation on the effect of a change of concentration of the electrolyte to the amount of the layer of copper deposited in an iron object iron spoon in an electroplating process. An example of electrolysis experiment 25 procedure as your instructor performs the experiment record observations in data table 1. Electrolysis and electroplating lab 4 chem 36 spring 2009 1 introduction reactions that involve a change in the number of electrons at a particular location require a reduction or oxidation process to take place. In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. An investigation on the effect of a change of concentration of the electrolyte to the amount of the layer of copper deposited in an iron object iron spoon in an electroplating process.
 Source: studylib.net
Source: studylib.net
Add 1 m cuso 4. This lab is designed to provide an example of the reduction oxidation redox processes. In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. Attach a wire to the copper strip. An investigation on the effect of a change of concentration of the electrolyte to the amount of the layer of copper deposited in an iron object iron spoon in an electroplating process.
 Source: davidswart.org
Source: davidswart.org
An example of electrolysis experiment 25 procedure as your instructor performs the experiment record observations in data table 1. In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. Current a an increase in the amount of current increases the amount of substance deposited in a fixed amount of time because there is more energy available to move ions electrons to the cathode from the anode. Clean a small strip of copper with steel wool. The lab report is due friday evening.
 Source: coursehero.com
Source: coursehero.com
In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. During electroplating a thin layer of a desirable metal is deposited onto another object. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current. In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. Current a an increase in the amount of current increases the amount of substance deposited in a fixed amount of time because there is more energy available to move ions electrons to the cathode from the anode.
 Source: studylib.net
Source: studylib.net
The lab report is due friday evening. Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current. In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. Electrolysis and electroplating lab 4 chem 36 spring 2009 1 introduction reactions that involve a change in the number of electrons at a particular location require a reduction or oxidation process to take place.
 Source: coursehero.com
Source: coursehero.com
During electroplating a thin layer of a desirable metal is deposited onto another object. Each student should use a different color font and team members should work together to determine how to equally divide the work. An example of electrolysis experiment 25 procedure as your instructor performs the experiment record observations in data table 1. Penny electroplating lab free download as word doc doc pdf file pdf text file txt or read online for free. The lab report is due friday evening.
 Source: manualzz.com
Source: manualzz.com
An example of electrolysis experiment 25 procedure as your instructor performs the experiment record observations in data table 1. Penny electroplating lab free download as word doc doc pdf file pdf text file txt or read online for free. A smaller current will pump fewer copper ions from the anode to the cathode in the same. The lab report is due friday evening. Clean a small piece of the object to be plated with steel wool.
 Source: studocu.com
Source: studocu.com
Penny electroplating lab free download as word doc doc pdf file pdf text file txt or read online for free. To conclude our electroplating lab students will collaborate with their lab team to write a team lab report shared with the teacher as a google doc. Clean a small strip of copper with steel wool. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry. Penny electroplating lab free download as word doc doc pdf file pdf text file txt or read online for free.
 Source: studylib.net
Source: studylib.net
A smaller current will pump fewer copper ions from the anode to the cathode in the same. This lab is designed to provide an example of the reduction oxidation redox processes. Current a an increase in the amount of current increases the amount of substance deposited in a fixed amount of time because there is more energy available to move ions electrons to the cathode from the anode. Attach wire to the object. Electrolysis and electroplating lab 4 chem 36 spring 2009 1 introduction reactions that involve a change in the number of electrons at a particular location require a reduction or oxidation process to take place.
 Source: markedbyteachers.com
Source: markedbyteachers.com
During electroplating a thin layer of a desirable metal is deposited onto another object. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry. To conclude our electroplating lab students will collaborate with their lab team to write a team lab report shared with the teacher as a google doc. Current a an increase in the amount of current increases the amount of substance deposited in a fixed amount of time because there is more energy available to move ions electrons to the cathode from the anode. Attach a wire to the copper strip.
Source: flinnsci.com
This lab is designed to provide an example of the reduction oxidation redox processes. An example of electrolysis experiment 25 procedure as your instructor performs the experiment record observations in data table 1. A 3 4 inch long niobium wire was obtained and cleaned to remove fingerprints. Attach a wire to the copper strip. Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects.
 Source: obica.jp
Source: obica.jp
Electochemistry electroplating overview electroplating is an economically important process often used to reduce corrosion or improve the appearance of objects. Add 1 m cuso 4. This lab is designed to provide an example of the reduction oxidation redox processes. An investigation on the effect of a change of concentration of the electrolyte to the amount of the layer of copper deposited in an iron object iron spoon in an electroplating process. Clean a small piece of the object to be plated with steel wool.

During electroplating a thin layer of a desirable metal is deposited onto another object. Penny electroplating lab free download as word doc doc pdf file pdf text file txt or read online for free. This lab is designed to provide an example of the reduction oxidation redox processes. Attach a wire to the copper strip. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry.

In the third experiment a beaker containing an aqueous borax solution a plastic mesh and a stainless steel wire strip was obtained. Clean a small strip of copper with steel wool. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry. A 3 4 inch long niobium wire was obtained and cleaned to remove fingerprints. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electroplating lab report by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





