Electroplating a coin with copper
Electroplating A Coin With Copper. The solution will turn blue indicating that the scrub has dissolved into the solution. The object being plated is referred to as the substrate. Electroplating is a common manufacturing method that involves applying a thin layer of metal onto another. Wear gloves from this step on because the copper solution is toxic.
Chem2u Electroplating A Coin With Copper From chem2u.blogspot.com
Throughout the lessons everybody paid 100 attention. This should leave a nice copper coating. Hook your piece of copper i used 1 8 but it doesn t need to be that heavy to the positive connection on your terminal using alligator clips and the negative side to your coin. Dry it off on a paper towel. This takes advantage of the fact that we are plating a metal object that conducts electricity. Beginner full instructions provided 2 hours 12 181.
This should leave a nice copper coating.
Schematic of an electroplating cell with a copper sulfate plating bath. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Beginner full instructions provided 2 hours 12 181. Dry it off on a paper towel. And submerge the coin all the way into the acid water mixture for approx ten minutes.
 Source: m.youtube.com
Source: m.youtube.com
This should leave a nice copper coating. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. Throughout the lessons everybody paid 100 attention. I started off by plating some coins. You can substitute the copper scoring pads for scrap copper pipe or wire.
Source: chem2u.blogspot.com
This takes advantage of the fact that we are plating a metal object that conducts electricity. Electroplating is a common manufacturing method that involves applying a thin layer of metal onto another. I plated each coin for about five to ten minutes and was. Rum aulia ananda y. And submerge the coin all the way into the acid water mixture for approx ten minutes.
 Source: chemistryhive.com
Source: chemistryhive.com
Wear gloves from this step on because the copper solution is toxic. These were done at around 3 volts and were pulling around 600ma during the plating process. In this video electroplating of coin is shown by the copper sulphate it gets copper coat while process diy copper chemical make. The object being plated is referred to as the substrate. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers.
Source: chem2u.blogspot.com
These were done at around 3 volts and were pulling around 600ma during the plating process. I started off by plating some coins. In this video electroplating of coin is shown by the copper sulphate it gets copper coat while process diy copper chemical make. And submerge the coin all the way into the acid water mixture for approx ten minutes. Schematic of an electroplating cell with a copper sulfate plating bath.
 Source: kiwico.com
Source: kiwico.com
Electroplate conductive materials using an arduino uno and a relay. Rois physical chemistry laboratory chemical engineering departement institut teknologi sepuluh nopember surabaya indonesia background after the invention of voltaic cell the cell was applied to electrolize water to produce hydrogen and oxygen. This should leave a nice copper coating. I plated each coin for about five to ten minutes and was. Electroplating a coin with copper yesterday when i entered my chemistry class i challenged my students to electroplate their key chains coins with copper before leaving chemistry laboratory at the end of my lessons.
 Source: test.scoilnet.ie
Source: test.scoilnet.ie
You can substitute the copper scoring pads for scrap copper pipe or wire. The object being plated is referred to as the substrate. Your solution should be dark blue. Rum aulia ananda y. Soak half of the copper scrubbing pad in the vinegar peroxide solution.
 Source: steemit.com
Source: steemit.com
By the time they started working in groups they started talking. Wear gloves from this step on because the copper solution is toxic. Electroplating a coin with copper yesterday when i entered my chemistry class i challenged my students to electroplate their key chains coins with copper before leaving chemistry laboratory at the end of my lessons. This should leave a nice copper coating. Electroplating is a common manufacturing method that involves applying a thin layer of metal onto another.
Source:
Electroplate conductive materials using an arduino uno and a relay. The solution will turn blue indicating that the scrub has dissolved into the solution. Your solution should be dark blue. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. Beginner full instructions provided 2 hours 12 181.
 Source: chemedx.org
Source: chemedx.org
Rum aulia ananda y. Electroplate conductive materials using an arduino uno and a relay. Soak half of the copper scrubbing pad in the vinegar peroxide solution. By the time they started working in groups they started talking. You now have a copper ion solution that can be used for electroplating.
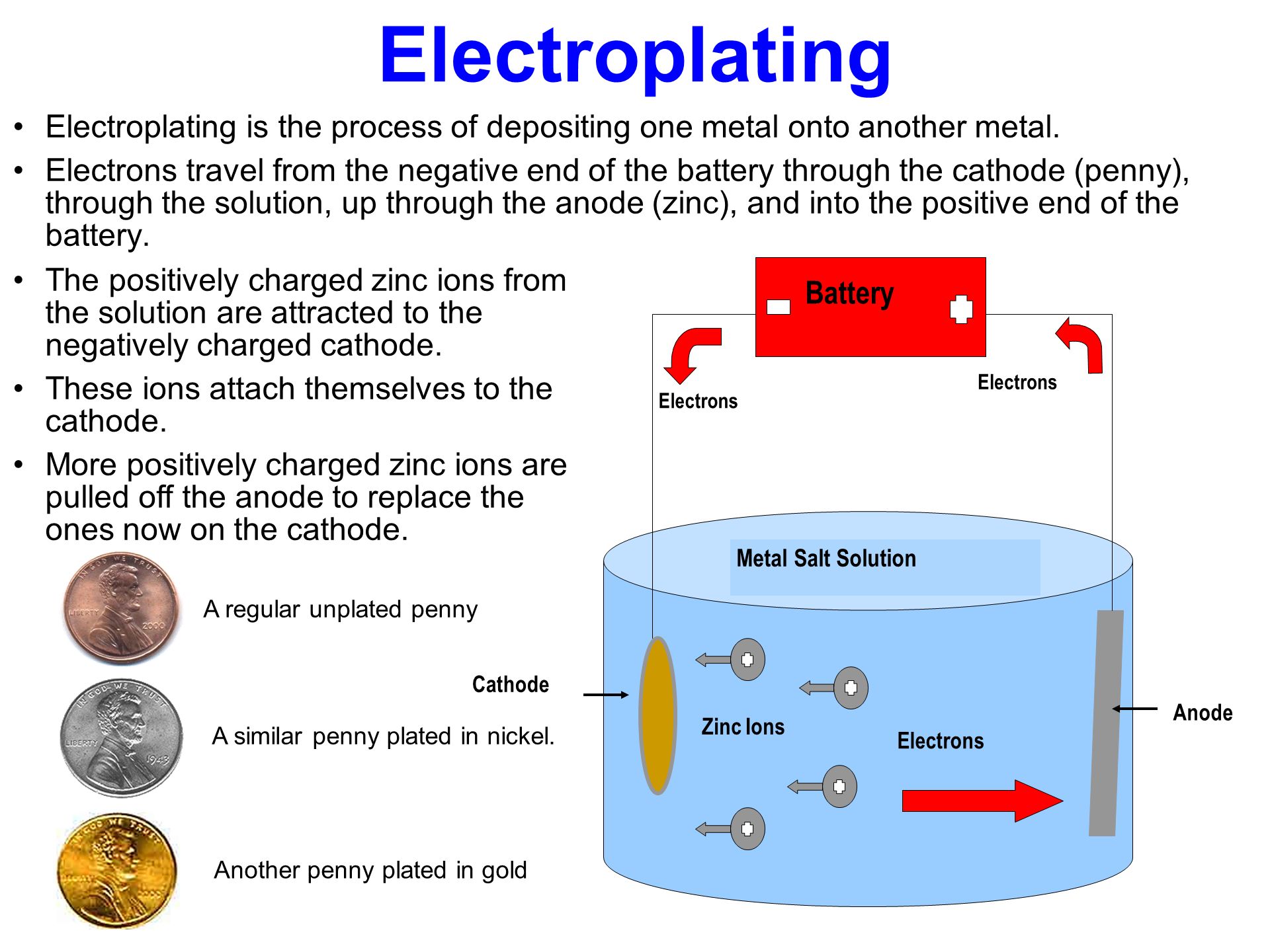 Source: slideplayer.com
Source: slideplayer.com
And submerge the coin all the way into the acid water mixture for approx ten minutes. Electroplate conductive materials using an arduino uno and a relay. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. I started off by plating some coins. By the time they started working in groups they started talking.
 Source: youtube.com
Source: youtube.com
Soak half of the copper scrubbing pad in the vinegar peroxide solution. Hook your piece of copper i used 1 8 but it doesn t need to be that heavy to the positive connection on your terminal using alligator clips and the negative side to your coin. Electroplating is a common manufacturing method that involves applying a thin layer of metal onto another. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Dry it off on a paper towel.
 Source: pinterest.com
Source: pinterest.com
The object being plated is referred to as the substrate. I plated each coin for about five to ten minutes and was. In this video electroplating of coin is shown by the copper sulphate it gets copper coat while process diy copper chemical make. The object being plated is referred to as the substrate. I started off by plating some coins.
Source:
Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. If you plan on electroplating very large things you will need to buy a lot of vinegar hydrogen peroxide copper and larger containers. Dry it off on a paper towel. Wear gloves from this step on because the copper solution is toxic. I started off by plating some coins.
 Source: youtube.com
Source: youtube.com
This should leave a nice copper coating. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. I plated each coin for about five to ten minutes and was. Your solution should be dark blue. Stir copper sulfate into some hot water in a beaker until no more will dissolve.
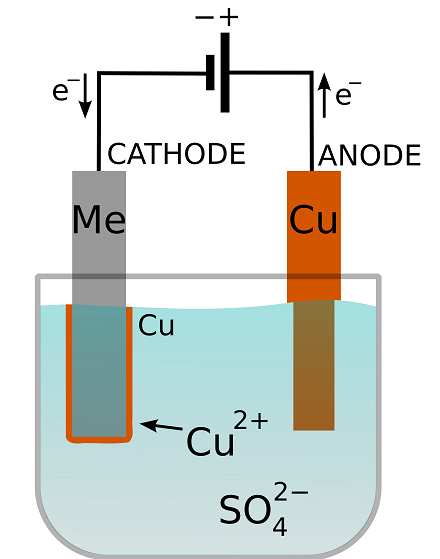 Source: comsol.com
Source: comsol.com
And submerge the coin all the way into the acid water mixture for approx ten minutes. Conversely you can cover a copper electrode in cotton batting dip it in the electrolyte solution and then paint the copper on. In this video electroplating of coin is shown by the copper sulphate it gets copper coat while process diy copper chemical make. This should leave a nice copper coating. Dry it off on a paper towel.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electroplating a coin with copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.