Electrolysis sodium sulfate
Electrolysis Sodium Sulfate. Cathode colorless the carbon produces purple color and there is bubble. And this solution contains neither ce o 2 nor ce h ions. Electrolysis of sodium sulphate. However the invention will be described in detail in connection with the electrolytic.
 Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
The contents of cell g is allowed to flow oil into cell h and dilute sodium sulphate solution is then led from the cell f into the empty cell. Study electrolysis experperiment 1 sodium sulfate solution flashcards from cathal o leary s class online or in brainscape s iphone or android app. However the invention will be described in detail in connection with the electrolytic. Electrolysis involves using electricity to break down electrolytes to form elements. Cathode colorless the carbon produces purple color and there is bubble. So all your chemical concepts are pure fantasy.
The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
And this solution contains neither ce o 2 nor ce h ions. Learn faster with spaced repetition. The contents of cell g is allowed to flow oil into cell h and dilute sodium sulphate solution is then led from the cell f into the empty cell. Aqa required practical for gcse combined science or chemsitry. The products of electrolysis can be predicted for a given electrolyte. Investigate what happens when aqueous solutions are electrolysed using inert electrodes.
 Source: chegg.com
Source: chegg.com
Cathode colorless the carbon produces purple color and there is bubble. Sulphuric acid is formed in the cells g m. So all your chemical concepts are pure fantasy. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble. Electrolysis of sodium sulphate.
 Source: chegg.com
Source: chegg.com
Cathode colorless the carbon produces purple color and there is bubble. Electrolysis simply means passing current through a solution containing ions. Electrolysis of sodium sulphate. As previously indicated aqueous solutions of any salt may be electrolyzed in accordance with the invention. Cathode colorless the carbon produces purple color and there is bubble.

Copper can be purified using electrolysis. Electrolysis simply means passing current through a solution containing ions. However the invention will be described in detail in connection with the electrolytic. Electrolysis involves using electricity to break down electrolytes to form elements. One of the solutions contains only dissolved sodium sulfate.
 Source: m.youtube.com
Source: m.youtube.com
So all your chemical concepts are pure fantasy. However the invention will be described in detail in connection with the electrolytic. And this solution contains neither ce o 2 nor ce h ions. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
 Source: docbrown.info
Source: docbrown.info
So all your chemical concepts are pure fantasy. However the invention will be described in detail in connection with the electrolytic. So all your chemical concepts are pure fantasy. Learn faster with spaced repetition. Aqa required practical for gcse combined science or chemsitry.
 Source: pt.slideshare.net
Source: pt.slideshare.net
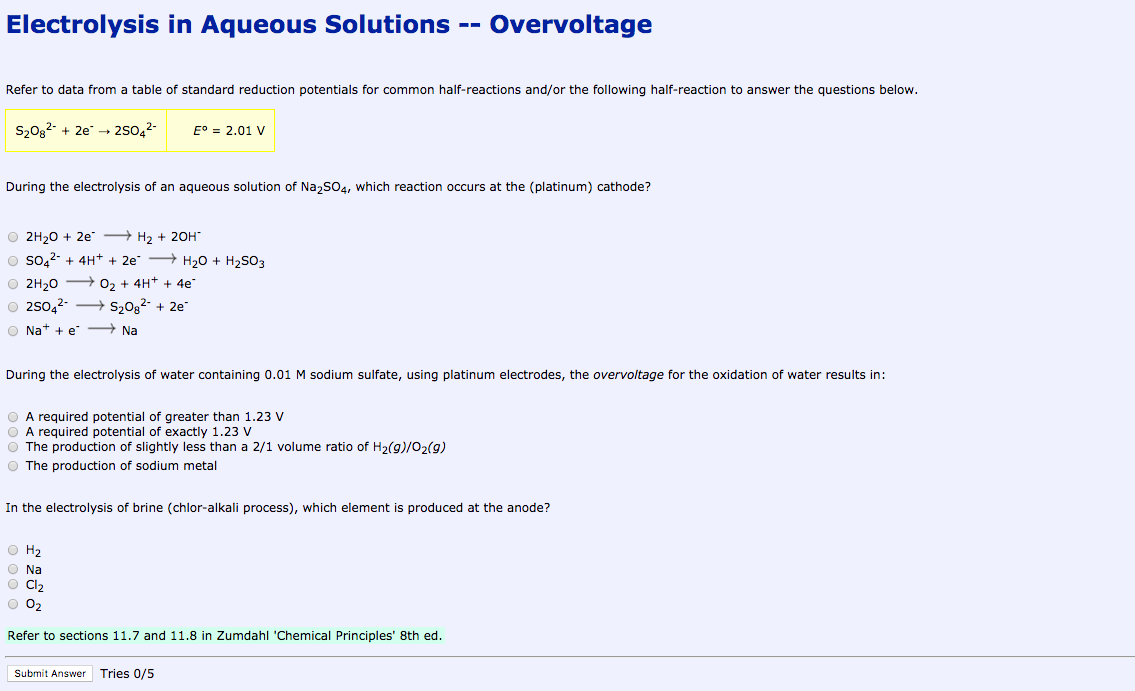
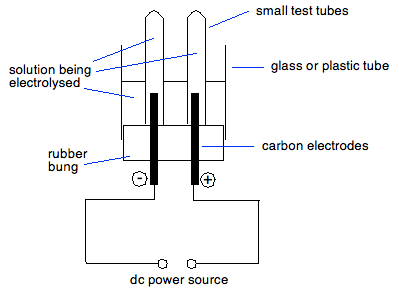
The h ions present in the are reduced at cathode and produces h 2 gas. The h ions present in the are reduced at cathode and produces h 2 gas. However the invention will be described in detail in connection with the electrolytic. In this part of the electrolysis of aqueous solutions lab you see the electrolysis of two aqueous sodium sulfate solutions. You will also see what happens when the electrolysis apparatus is placed in distilled water.
 Source: youtube.com
Source: youtube.com
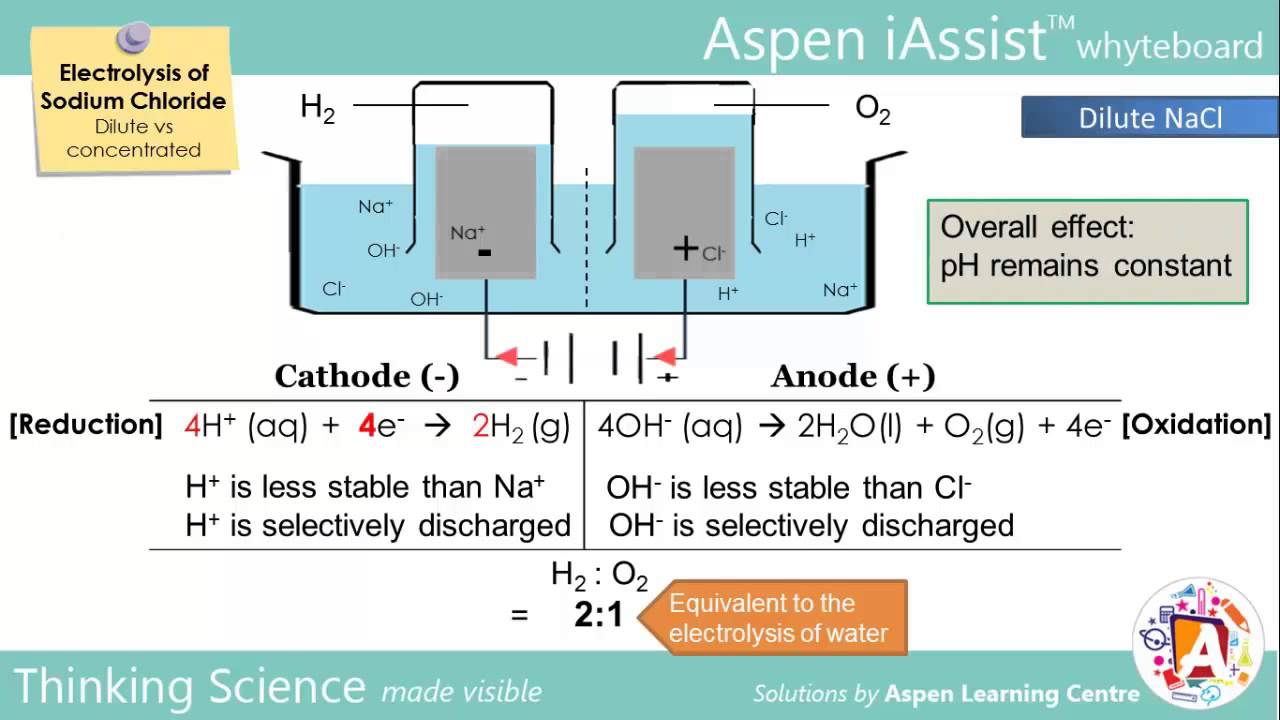
When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Bromthymol blue btb has been added to the other solution. Electrolysis involves using electricity to break down electrolytes to form elements. Copper can be purified using electrolysis. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
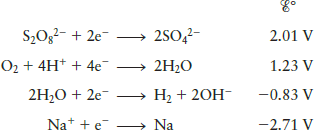 Source: chegg.com
Source: chegg.com
In this part of the electrolysis of aqueous solutions lab you see the electrolysis of two aqueous sodium sulfate solutions. The products of electrolysis can be predicted for a given electrolyte. The h ions present in the are reduced at cathode and produces h 2 gas. And this solution contains neither ce o 2 nor ce h ions. The electrolysis of sodium sulfate na2so4 data collection the side of anode and cathode the color before electrolysis the changes during electrolysis anode colorless the color stays the same and there is bubble.
 Source: aplustopper.com
Source: aplustopper.com
Aqa required practical for gcse combined science or chemsitry. Study electrolysis experperiment 1 sodium sulfate solution flashcards from cathal o leary s class online or in brainscape s iphone or android app. The products of electrolysis can be predicted for a given electrolyte. In this part of the electrolysis of aqueous solutions lab you see the electrolysis of two aqueous sodium sulfate solutions. As previously indicated aqueous solutions of any salt may be electrolyzed in accordance with the invention.
 Source: docbrown.info
Source: docbrown.info
Electrolysis involves using electricity to break down electrolytes to form elements. Study electrolysis experperiment 1 sodium sulfate solution flashcards from cathal o leary s class online or in brainscape s iphone or android app. The h ions present in the are reduced at cathode and produces h 2 gas. Investigate what happens when aqueous solutions are electrolysed using inert electrodes. However the invention will be described in detail in connection with the electrolytic.
 Source: slideplayer.com
Source: slideplayer.com
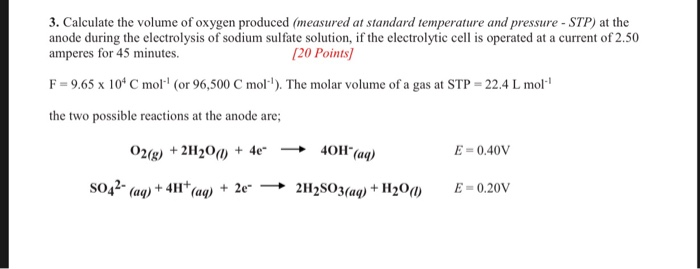
The electrolysis of a ce na2so4 solution does not produce any sulfur. Copper can be purified using electrolysis. The products of electrolysis can be predicted for a given electrolyte. The h ions present in the are reduced at cathode and produces h 2 gas. The contents of cell g is allowed to flow oil into cell h and dilute sodium sulphate solution is then led from the cell f into the empty cell.
 Source: markedbyteachers.com
Source: markedbyteachers.com
Aqa required practical for gcse combined science or chemsitry. And this solution contains neither ce o 2 nor ce h ions. One of the solutions contains only dissolved sodium sulfate. Aqa required practical for gcse combined science or chemsitry. Study electrolysis experperiment 1 sodium sulfate solution flashcards from cathal o leary s class online or in brainscape s iphone or android app.
 Source: tuttee.co
Source: tuttee.co
You will also see what happens when the electrolysis apparatus is placed in distilled water. The contents of cell g is allowed to flow oil into cell h and dilute sodium sulphate solution is then led from the cell f into the empty cell. In this part of the electrolysis of aqueous solutions lab you see the electrolysis of two aqueous sodium sulfate solutions. Study electrolysis experperiment 1 sodium sulfate solution flashcards from cathal o leary s class online or in brainscape s iphone or android app. Electrolysis involves using electricity to break down electrolytes to form elements.
 Source: chemguide.co.uk
Source: chemguide.co.uk
The products of electrolysis can be predicted for a given electrolyte. Sulphuric acid is formed in the cells g m. When the aqueous solution of sodium chloride is electrolysed the reduction and oxidation of the ions takes place at cathode and anode respectively. Aqa required practical for gcse combined science or chemsitry. And this solution contains neither ce o 2 nor ce h ions.
 Source: slideplayer.com
Source: slideplayer.com
Electrolysis simply means passing current through a solution containing ions. Electrolysis of sodium sulphate. Learn faster with spaced repetition. Sulphuric acid is formed in the cells g m. Cathode colorless the carbon produces purple color and there is bubble.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis sodium sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.