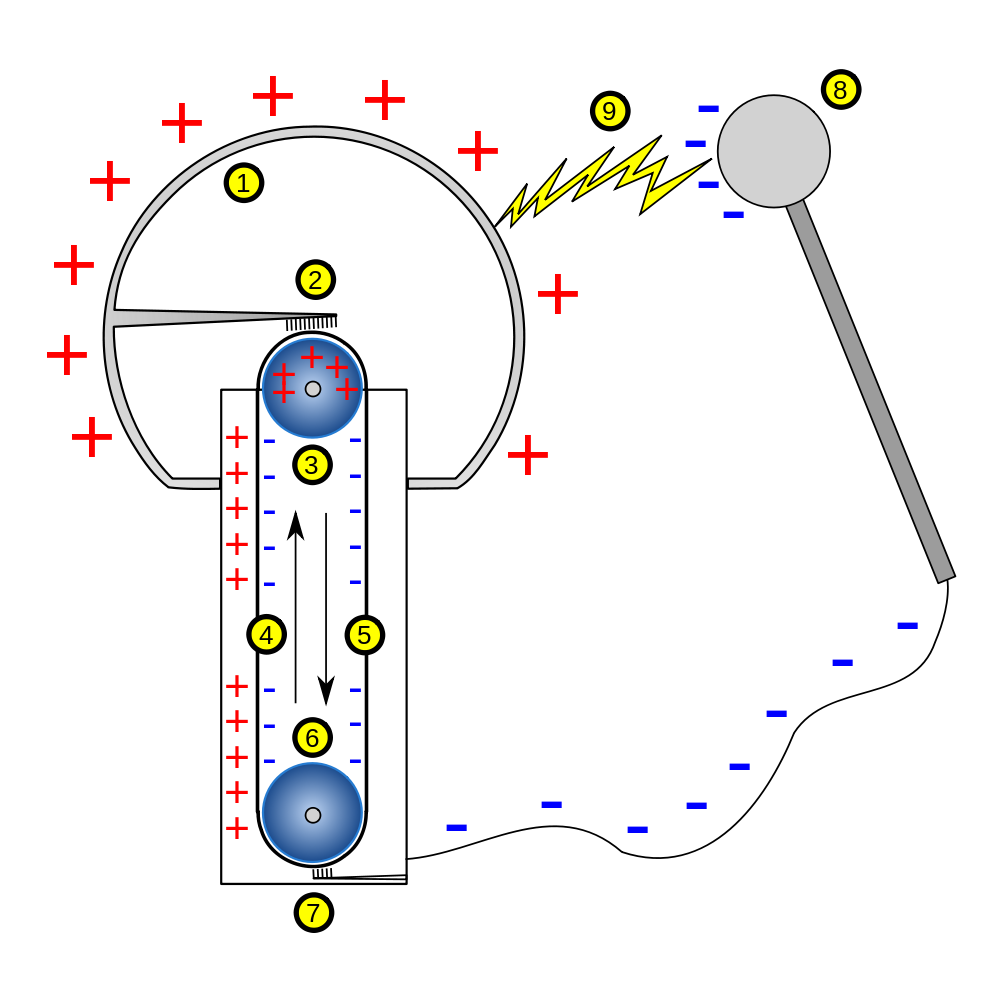
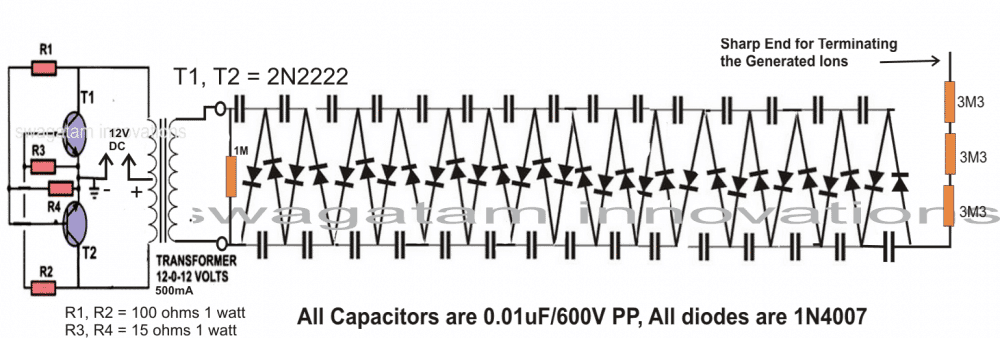
Electrolysis set up
Electrolysis Set Up. You ll need a plastic tub 2 test tubes 3 large rubber bands baking soda duct tape 9 volt batter. The solution eventually becomes nitric acid solution. This is called the cathode. Electrolysis is the process by which ionic substances are decomposed broken down into simpler substances when an electric current is passed through them.
Experimental Setup Used For Electrolysis Download Scientific Diagram From researchgate.net
Hydrogen ions and nitrate ions remain in the solution. This is one simple way to perform electrolysis of water. Electrolytic cell is also known as a voltameter since it generates voltage or current at its two terminals. In this case both hydrogen and oxygen exist in a gaseous state. The complete set up for electrolysis is called the electrolytic cell. In order to perform electrolysis you must run an electric current through water that contains an.
Hydrogen ions and nitrate ions remain in the solution.
To silver plate an object like a spoon plated silverware is less expensive than pure silver the spoon is placed in the position of the cathode in an electrolysis set up with a solution of silver nitrate. When the circuit in the set up shown below is closed the acidified potassium permanganate solution loses its color gradually. This is one simple way to perform electrolysis of water. In other words you break apart the molecules that make up water to restore the elements to their original state. Electrolytic cell is also known as a voltameter since it generates voltage or current at its two terminals. With power turned off attach the negative lead also called neutral or ground of the power source to the rusted piece that you want to restore.
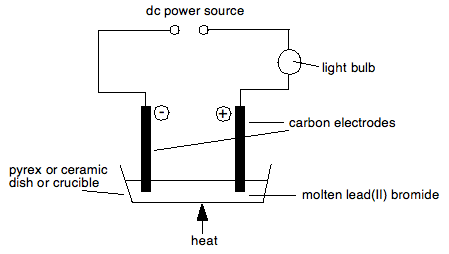 Source: chemguide.co.uk
Source: chemguide.co.uk
The solution eventually becomes nitric acid solution. This article will provide elaborate instructions on performing this procedure to get the best oxidation free results. That reaction has a potential of 2 06 v at standard conditions. The solution eventually becomes nitric acid solution. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it.
Source: researchgate.net
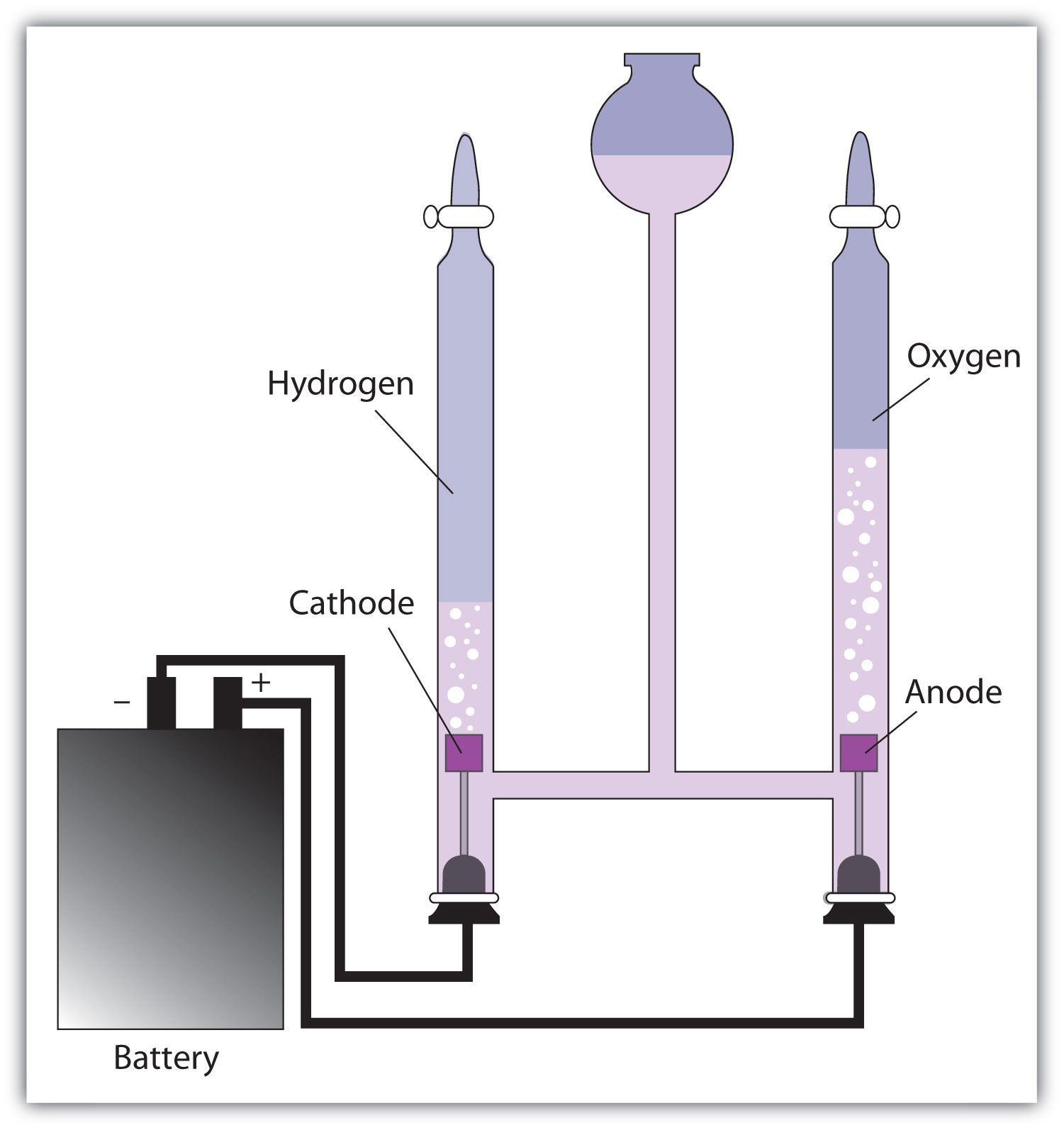
This is one simple way to perform electrolysis of water. With power turned off attach the negative lead also called neutral or ground of the power source to the rusted piece that you want to restore. In other words you break apart the molecules that make up water to restore the elements to their original state. This is called the cathode. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion.

The solution eventually becomes nitric acid solution. The solution eventually becomes nitric acid solution. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. Silver ions and hydroxide ions are consumed in the electrolysis. This consists of the vessel containing the electrolyte anode cathode battery and wires.
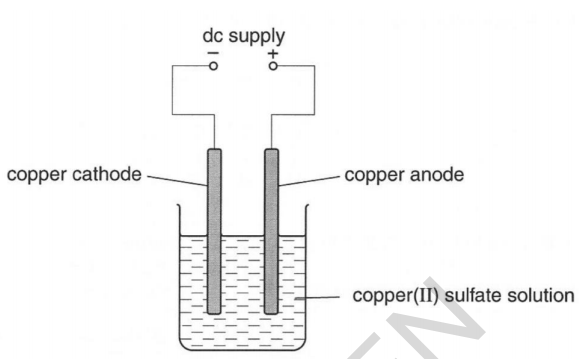 Source: elevise.co.uk
Source: elevise.co.uk
In other words you break apart the molecules that make up water to restore the elements to their original state. This article will provide elaborate instructions on performing this procedure to get the best oxidation free results. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. Electrolysis is the process by which ionic substances are decomposed broken down into simpler substances when an electric current is passed through them. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it.
 Source: researchgate.net
Source: researchgate.net
Silver ions and hydroxide ions are consumed in the electrolysis. When the circuit in the set up shown below is closed the acidified potassium permanganate solution loses its color gradually. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it. In order to perform electrolysis you must run an electric current through water that contains an. Place the piece in the middle of the container.
Source: researchgate.net
This article will provide elaborate instructions on performing this procedure to get the best oxidation free results. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it. Electrolytic cell is also known as a voltameter since it generates voltage or current at its two terminals. To silver plate an object like a spoon plated silverware is less expensive than pure silver the spoon is placed in the position of the cathode in an electrolysis set up with a solution of silver nitrate. This article will provide elaborate instructions on performing this procedure to get the best oxidation free results.
 Source: pinterest.cl
Source: pinterest.cl
Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. Silver ions and hydroxide ions are consumed in the electrolysis. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it. This is one simple way to perform electrolysis of water. With power turned off attach the negative lead also called neutral or ground of the power source to the rusted piece that you want to restore.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
In this case both hydrogen and oxygen exist in a gaseous state. Electrolysis setup with sacrificial anode cathode restoration and plastic container. With power turned off attach the negative lead also called neutral or ground of the power source to the rusted piece that you want to restore. In other words you break apart the molecules that make up water to restore the elements to their original state. The solution eventually becomes nitric acid solution.
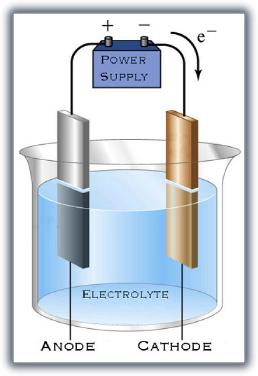 Source: rustyiron.com
Source: rustyiron.com
This consists of the vessel containing the electrolyte anode cathode battery and wires. In order to perform electrolysis you must run an electric current through water that contains an. The process is quite easy to do and requires very basic equipment. The solution eventually becomes nitric acid solution. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion.
Source: researchgate.net
This is one simple way to perform electrolysis of water. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. Electrolysis is a very effective method for removing rust from ferrous items without causing noticeable damage. You ll need a plastic tub 2 test tubes 3 large rubber bands baking soda duct tape 9 volt batter. In other words you break apart the molecules that make up water to restore the elements to their original state.
 Source: msnucleus.org
Source: msnucleus.org
Electrolysis setup with sacrificial anode cathode restoration and plastic container. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it. This is called the cathode. Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. The complete set up for electrolysis is called the electrolytic cell.

Place the piece in the middle of the container. In this case both hydrogen and oxygen exist in a gaseous state. The solution eventually becomes nitric acid solution. You ll need a plastic tub 2 test tubes 3 large rubber bands baking soda duct tape 9 volt batter. The process is quite easy to do and requires very basic equipment.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
Electrolytic cell is also known as a voltameter since it generates voltage or current at its two terminals. When the current is turned on the silver ions will migrate through the solution touch the cathode spoon and adhere to it. This is one simple way to perform electrolysis of water. With power turned off attach the negative lead also called neutral or ground of the power source to the rusted piece that you want to restore. Place the piece in the middle of the container.
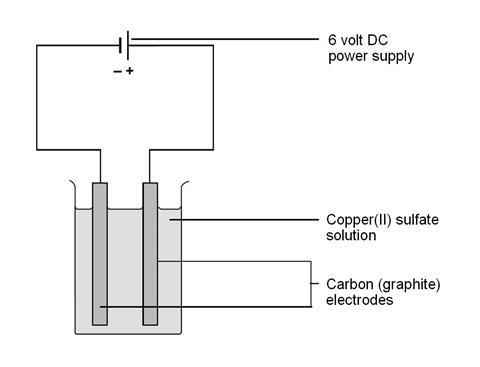 Source: edu.rsc.org
Source: edu.rsc.org
The solution eventually becomes nitric acid solution. In other words you break apart the molecules that make up water to restore the elements to their original state. Electrolysis setup with sacrificial anode cathode restoration and plastic container. This consists of the vessel containing the electrolyte anode cathode battery and wires. Electrolytic cell is also known as a voltameter since it generates voltage or current at its two terminals.
 Source: year11misadventures.blogspot.com
Source: year11misadventures.blogspot.com
Setup for the electrolysis of water the reaction at the anode is the oxidation of water to o 2 and acid while the cathode reduces water into h 2 and hydroxide ion. Electrolysis is the process by which ionic substances are decomposed broken down into simpler substances when an electric current is passed through them. The solution eventually becomes nitric acid solution. When the circuit in the set up shown below is closed the acidified potassium permanganate solution loses its color gradually. Electrolysis setup with sacrificial anode cathode restoration and plastic container.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis set up by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.