Electrolysis of water lab
Electrolysis Of Water Lab. 6 or 9 volt battery. To determine the ratio in which elements form water. Passing electricity through water will split water into oxygen and hydrogen. Fill the large beaker approximately three quarters full with water.

6 volt or 9 volt battery. Electrolysis of water lab purpose. During electrolysis electrical energy is used to cause a nonspontaneous chemical reaction to occur. Invert the test tubes placing the open tops under water in the large jar. You may find the next step easier if you cover the open ends of the test tube with a small piece of paper. By adding sulfuric acid to the water it will speed up the reaction because it s an electrolyte.
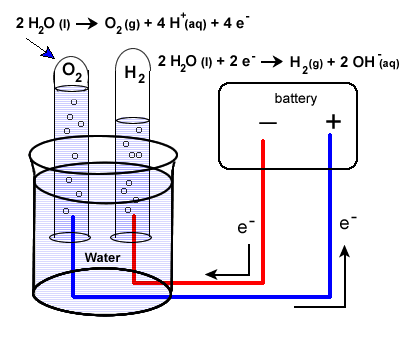
On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes.
Two alligator clip leads or insulated wire. An electrolysis of water experiment and an electroplating experiment electrolysis. Electrolysis of water lab introduction. By adding sulfuric acid to the water it will speed up the reaction because it s an electrolyte. 6 volt or 9 volt battery. For this experiment you can gather your own supplies or buy a complete water electrolysis kit.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Two alligator clip leads or insulated wire. Electrolysis of water lab introduction. For this experiment you can gather your own supplies or buy a complete water electrolysis kit. 6 or 9 volt battery. Table salt or sodium chloride nacl is also a good additive to form electrolytes.
 Source: youtube.com
Source: youtube.com
Piece of thin cardstock or cardboard. Electrolysis of water lab introduction. Passing electricity through water will split water into oxygen and hydrogen. By adding sulfuric acid to the water it will speed up the reaction because it s an electrolyte. 6 or 9 volt battery.
 Source: pinterest.com
Source: pinterest.com
Two alligator clip leads or insulated wire. Water electrolysis splitting experiment hypothesis. Often used to obtain elements that are too. 6 or 9 volt battery. Fill the large beaker approximately three quarters full with water.
 Source: en.wikipedia.org
Source: en.wikipedia.org
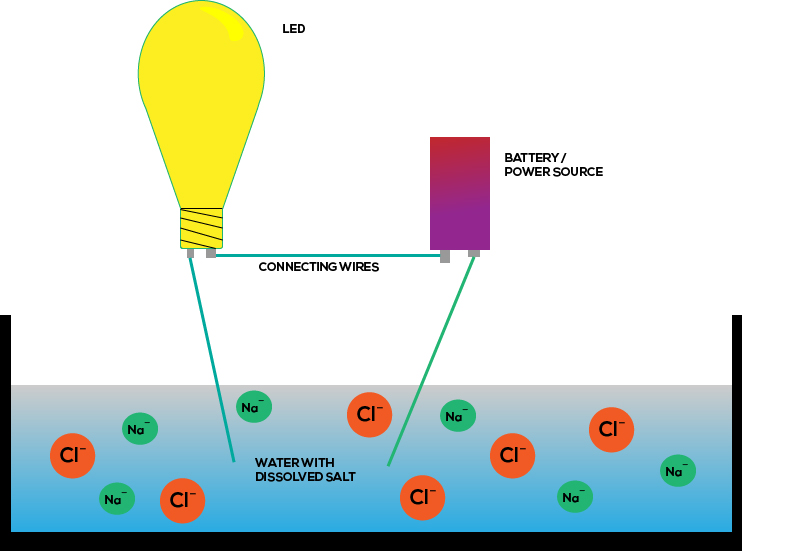
Electrochemistry is the study of the relationship between electrical forces and chemical reactions. To determine the ratio in which elements form water. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric. Invert the test tubes placing the open tops under water in the large jar. Two insulated wires or alligator clip leads.

Often used to obtain elements that are too. Passing electricity through water will split water into oxygen and hydrogen. Invert the test tubes placing the open tops under water in the large jar. Table salt or sodium chloride nacl is also a good additive to form electrolytes. Often used to obtain elements that are too.
 Source: thinkswap.com
Source: thinkswap.com
Two insulated wires or alligator clip leads. Often used to obtain elements that are too. Two insulated wires or alligator clip leads. To determine the ratio in which elements form water. 6 volt or 9 volt battery.
 Source: slideshare.net
Source: slideshare.net
Invert the test tubes placing the open tops under water in the large jar. Piece of thin cardboard or card stock. Two insulated wires or alligator clip leads. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes. An electrolysis of water experiment and an electroplating experiment electrolysis.
 Source: studylib.net
Source: studylib.net
Electrochemistry is the study of the relationship between electrical forces and chemical reactions. You may find the next step easier if you cover the open ends of the test tube with a small piece of paper. 6 volt or 9 volt battery. Passing electricity through water will split water into oxygen and hydrogen. An electrolysis of water experiment and an electroplating experiment electrolysis.
 Source: sites.prairiesouth.ca
Source: sites.prairiesouth.ca
You may find the next step easier if you cover the open ends of the test tube with a small piece of paper. Piece of thin cardboard or card stock. During electrolysis electrical energy is used to cause a nonspontaneous chemical reaction to occur. Water electrolysis splitting experiment hypothesis. Often used to obtain elements that are too.

For this experiment you can gather your own supplies or buy a complete water electrolysis kit. Two alligator clip leads or insulated wire. 6 or 9 volt battery. Fill the large beaker approximately three quarters full with water. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes.
 Source: kidslovekits.com
Source: kidslovekits.com
In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. On passing electricity through water it splits or electrolyzes to give off hydrogen and oxygen gases at the two electrodes. For this experiment you can gather your own supplies or buy a complete water electrolysis kit. Piece of thin cardstock or cardboard. Electrolysis of water lab introduction.
 Source: pinterest.com
Source: pinterest.com
Table salt or sodium chloride nacl is also a good additive to form electrolytes. Table salt or sodium chloride nacl is also a good additive to form electrolytes. Electrochemistry is the study of the relationship between electrical forces and chemical reactions. Electrolysis of water lab purpose. Often used to obtain elements that are too.
 Source: nigerianscholars.com
Source: nigerianscholars.com
An electrolysis of water experiment and an electroplating experiment electrolysis. By adding sulfuric acid to the water it will speed up the reaction because it s an electrolyte. In a voltaic cell commonly known as a battery the. Passing electricity through water will split water into oxygen and hydrogen. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2.
 Source: studylib.net
Source: studylib.net
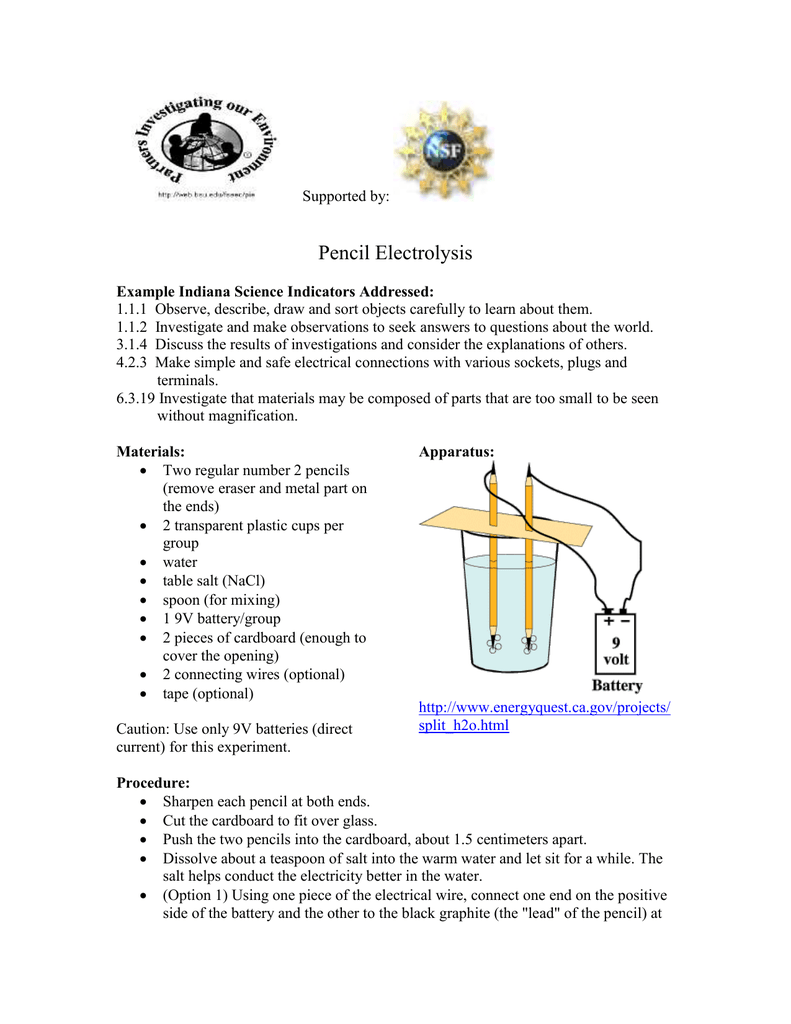
Two alligator clip leads or insulated wire. Fill the large beaker approximately three quarters full with water. Water sulfuric acid power supply 2x electrodes beaker 2x test tube rubber gasket scoopula peg board 2x. Water electrolysis splitting experiment hypothesis. Piece of thin cardboard or card stock.
 Source: scienceprojectideas.org
Source: scienceprojectideas.org
Piece of thin cardboard or card stock. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. Electrolysis of water lab introduction. To determine the ratio in which elements form water. Often used to obtain elements that are too.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis of water lab by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.