Electrolysis of water formula
Electrolysis Of Water Formula. Z is the electrochemical equivalent which is the mass of a substance produced at the electrode during electrolysis by. Water is a poor conductor of electricity but it does contain some hydrogen ions h and hydroxide ions oh. The concentration of the ions in neutral water are equal moles per litre. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications.
During The Electrolysis Of Water How Many Moles Of Oxygen Are Produced When 20 Moles Of Water Are Decomposed Quora From quora.com
So water with a very small amount of ions is a bad conductor of electricity. Z is the electrochemical equivalent which is the mass of a substance produced at the electrode during electrolysis by. So electrolysis of pure water will be a very slow process. The decomposition of water produces twice as much hydrogen gas as oxygen gas. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. By adding ordinary table salt nacl to distilled water it becomes an electrolyte solution able to conduct electricity.
Water is very weakly dissociated into hydrogen and hydroxide ions.
We have just used that without actually stating it it is basically obvious. So electrolysis of pure water will be a very slow process. So water with a very small amount of ions is a bad conductor of electricity. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries. The amount of charge transferred during electrolysis can be calculated from the mean current used and the time taken. Z is the electrochemical equivalent which is the mass of a substance produced at the electrode during electrolysis by.
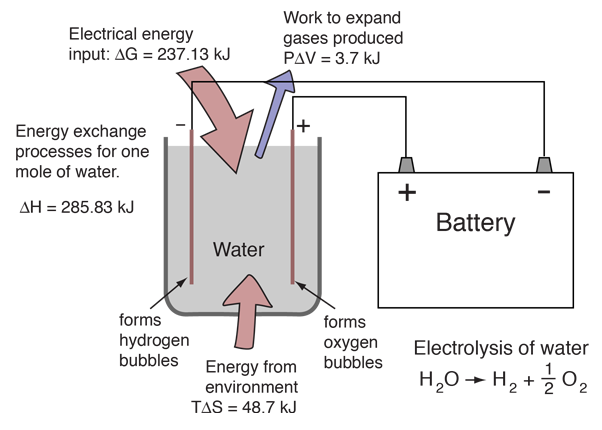 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
So water with a very small amount of ions is a bad conductor of electricity. So electrolysis of pure water will be a very slow process. Water is a poor conductor of electricity but it does contain some hydrogen ions h and hydroxide ions oh. These ions are formed when a small proportion of water molecules naturally dissociate. Hydrogen h2 oxygen o what is the conclusion of electrolysis of water.
 Source: highschoolenergy.acs.org
Source: highschoolenergy.acs.org
By adding ordinary table salt nacl to distilled water it becomes an electrolyte solution able to conduct electricity. Thus it is a very poor conductor of electricity. So electrolysis of pure water will be a very slow process. Water is comprised of two elements hydrogen h and oxygen o. The amount of charge transferred during electrolysis can be calculated from the mean current used and the time taken.
 Source: essentialchemicalindustry.org
Source: essentialchemicalindustry.org
Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Electrolysis of water by providing energy from a battery water h 2 o can be dissociated into the diatomic molecules of hydrogen h 2 and oxygen o 2 this process is a good example of the the application of the four thermodynamic potentials. By adding ordinary table salt nacl to distilled water it becomes an electrolyte solution able to conduct electricity. So electrolysis of pure water will be a very slow process. So water with a very small amount of ions is a bad conductor of electricity.
Source: quora.com
Distilled water is pure and free of salts. Since the number of protons and hydroxide ions formed in the reaction are the same when the solutions are combined at the end of the electrolysis the indicator has the characteristic green color of a neutral solution. Charge q current i time t coulombs c amperes a seconds s. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries. So water with a very small amount of ions is a bad conductor of electricity.
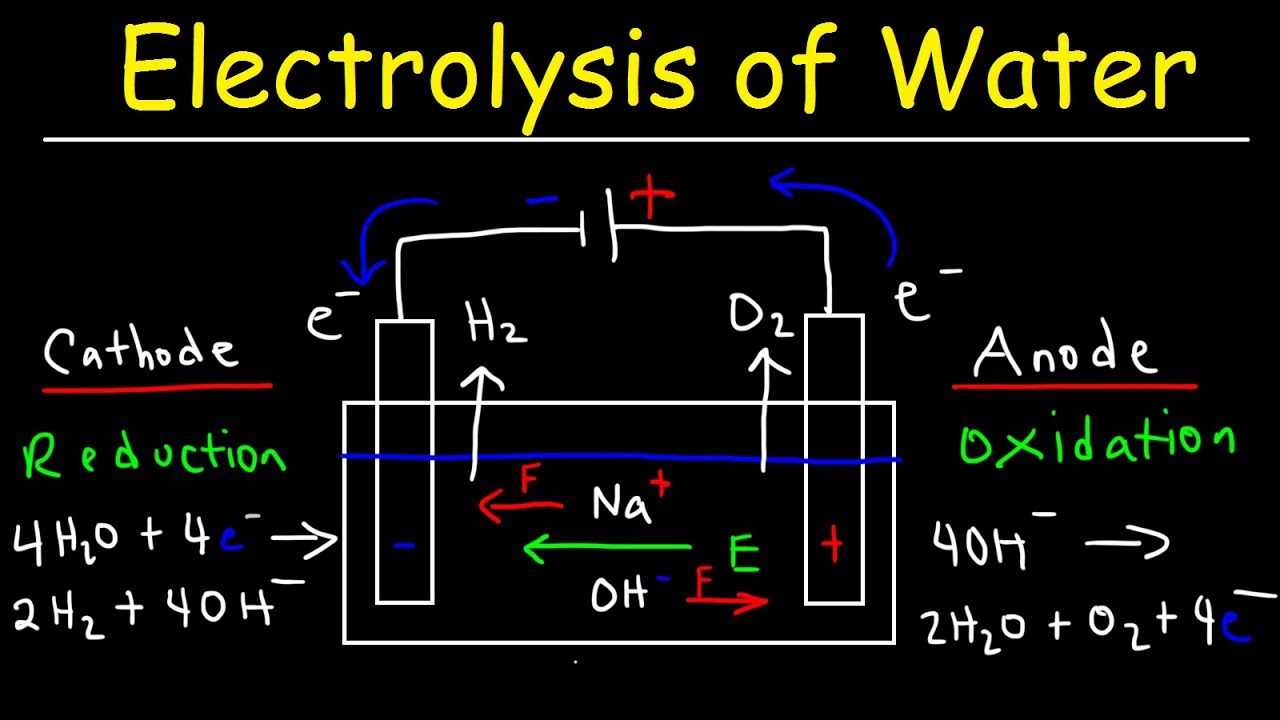 Source: youtube.com
Source: youtube.com
Since the number of protons and hydroxide ions formed in the reaction are the same when the solutions are combined at the end of the electrolysis the indicator has the characteristic green color of a neutral solution. The amount of charge transferred during electrolysis can be calculated from the mean current used and the time taken. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Electrolysis of water by providing energy from a battery water h 2 o can be dissociated into the diatomic molecules of hydrogen h 2 and oxygen o 2 this process is a good example of the the application of the four thermodynamic potentials.
Source: en.wikipedia.org
The electrolysis of one mole of water produces a mole of hydrogen gas and a half mole of oxygen gas in their normal diatomic forms. The concentration of the ions in neutral water are equal moles per litre. Water is a poor conductor of electricity but it does contain some hydrogen ions h and hydroxide ions oh. The amount of charge transferred during electrolysis can be calculated from the mean current used and the time taken. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications.
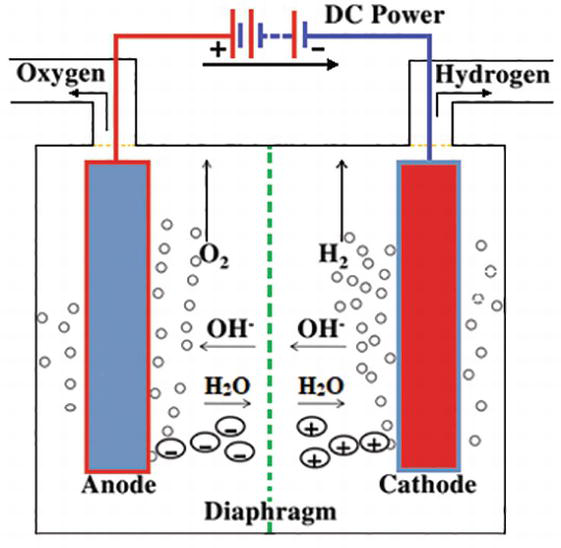 Source: intechopen.com
Source: intechopen.com
Since the number of protons and hydroxide ions formed in the reaction are the same when the solutions are combined at the end of the electrolysis the indicator has the characteristic green color of a neutral solution. The amount of charge transferred during electrolysis can be calculated from the mean current used and the time taken. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries. Electrolysis of water produces oxygen gas and hydrogen gas. Charge q current i time t coulombs c amperes a seconds s.
 Source: youtube.com
Source: youtube.com
So electrolysis of pure water will be a very slow process. So water with a very small amount of ions is a bad conductor of electricity. So electrolysis of pure water will be a very slow process. Thus it is a very poor conductor of electricity. The concentration of the ions in neutral water are equal moles per litre.
Source:
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Hydrogen h2 oxygen o what is the conclusion of electrolysis of water. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. By adding ordinary table salt nacl to distilled water it becomes an electrolyte solution able to conduct electricity. So water with a very small amount of ions is a bad conductor of electricity.
 Source: en.wikipedia.org
Source: en.wikipedia.org
So water with a very small amount of ions is a bad conductor of electricity. Water is comprised of two elements hydrogen h and oxygen o. The decomposition of water produces twice as much hydrogen gas as oxygen gas. Hydrogen h2 oxygen o what is the conclusion of electrolysis of water. So water with a very small amount of ions is a bad conductor of electricity.
 Source: docbrown.info
Source: docbrown.info
Since the number of protons and hydroxide ions formed in the reaction are the same when the solutions are combined at the end of the electrolysis the indicator has the characteristic green color of a neutral solution. So electrolysis of pure water will be a very slow process. Water is comprised of two elements hydrogen h and oxygen o. Distilled water is pure and free of salts. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries.
 Source: sciencedirect.com
Source: sciencedirect.com
Since the number of protons and hydroxide ions formed in the reaction are the same when the solutions are combined at the end of the electrolysis the indicator has the characteristic green color of a neutral solution. These ions are formed when a small proportion of water molecules naturally dissociate. The concentration of the ions in neutral water are equal moles per litre. Electrolysis involves the charge carriers for the current to flow. Distilled water is pure and free of salts.
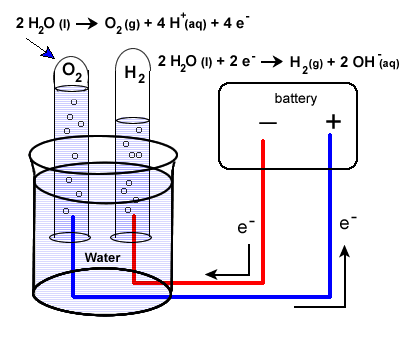 Source: sites.prairiesouth.ca
Source: sites.prairiesouth.ca
Thus it is a very poor conductor of electricity. Hydrogen h2 oxygen o what is the conclusion of electrolysis of water. These ions are formed when a small proportion of water molecules naturally dissociate. The electrolysis of one mole of water produces a mole of hydrogen gas and a half mole of oxygen gas in their normal diatomic forms. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries.

The decomposition of water produces twice as much hydrogen gas as oxygen gas. These ions are formed when a small proportion of water molecules naturally dissociate. Water is comprised of two elements hydrogen h and oxygen o. Thus it is a very poor conductor of electricity. Distilled water is pure and free of salts.
 Source: sciencedirect.com
Source: sciencedirect.com
By adding ordinary table salt nacl to distilled water it becomes an electrolyte solution able to conduct electricity. Water is comprised of two elements hydrogen h and oxygen o. Water is a poor conductor of electricity but it does contain some hydrogen ions h and hydroxide ions oh. The electrolysis of one mole of water produces a mole of hydrogen gas and a half mole of oxygen gas in their normal diatomic forms. You may come across the formula f le where f is the faraday constant l is the avogadro constant and e is the charge on an electron in terms of the number of coulombs it carries.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis of water formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






