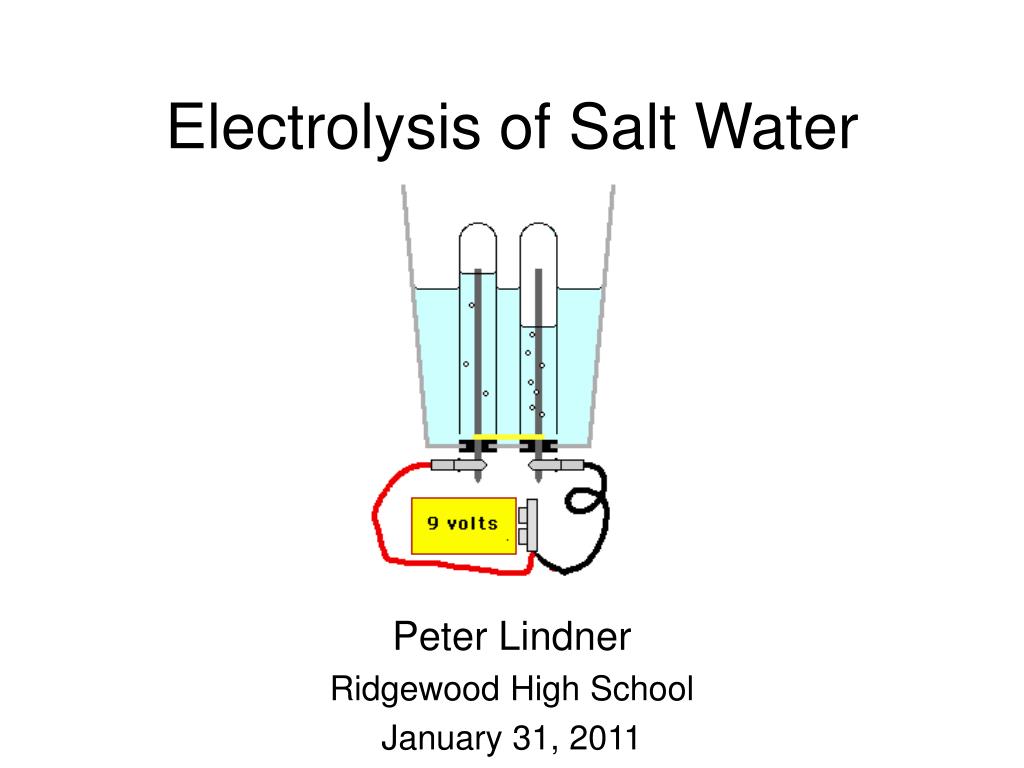
Electrolysis of saltwater
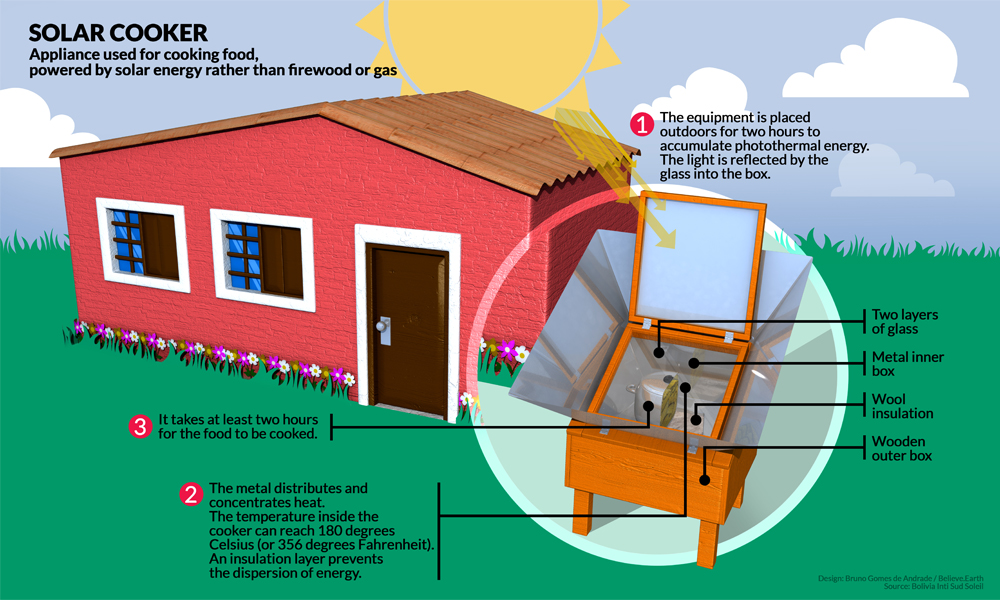
Electrolysis Of Saltwater. Two options exist for performance of this electrolysis. Chloride is negatively charged and is attracted to the positive side of the electrical charge where it bonds with oxygen and hydrogen from the water. This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells. Salt water chlorination is a process that uses dissolved salt 1000 36 000 ppm or 1 36 g l for the chlorination of swimming pools and hot tubs.
 Salt Waterswimming Pools From cascade.co.nz
Salt Waterswimming Pools From cascade.co.nz
The right hand side represents the products. Chloride is negatively charged and is attracted to the positive side of the electrical charge where it bonds with oxygen and hydrogen from the water. Water splitting with electricity electrolysis is a simple and old idea. Salt water chlorination is a process that uses dissolved salt 1000 36 000 ppm or 1 36 g l for the chlorination of swimming pools and hot tubs. The first option is to subject the water to total desalinization to remove all impurities and produce essentially distilled water. The left hand side represents the reactants.
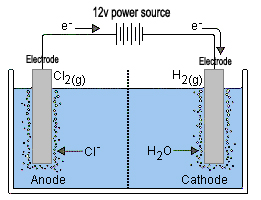
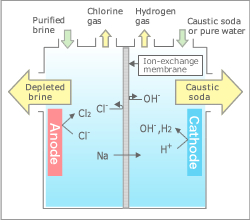
Naoh sodium hydroxide solution in the catholytic section the cathodic solution hydrogen gas which is given off at the cathode.
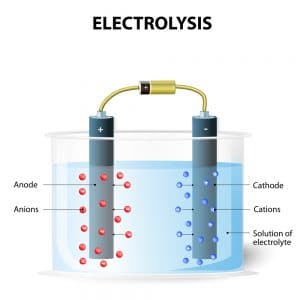
Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. So far so good. The left hand side represents the reactants. How do you create electrolyzed salt water. Electrolysis of an aqueous solution of table salt nacl or sodium chloride produces aqueous sodium hydroxide and chlorine although usually only in minute amounts. Common salt dissolved in water to form brine.
 Source: youtube.com
Source: youtube.com
How do you create electrolyzed salt water. Working in groups learners will. The left hand side represents the reactants. Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. So far so good.
 Source: slideserve.com
Source: slideserve.com
When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. Salt electrochlorination is through electrolysis of salt water or sea water in the anode produced by the reaction of chlorine gas and water in the solution of hypochlorite with a certain degree of disinfection and sterilization effect. This process is called electrolysis. The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are. Salt water chlorination is a process that uses dissolved salt 1000 36 000 ppm or 1 36 g l for the chlorination of swimming pools and hot tubs.
Source:
Naoh sodium hydroxide solution in the catholytic section the cathodic solution hydrogen gas which is given off at the cathode. Electrolysis of an aqueous solution of table salt nacl or sodium chloride produces aqueous sodium hydroxide and chlorine although usually only in minute amounts. When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics. The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics. Water splitting with electricity electrolysis is a simple and old idea. Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. In the process of salt electrochlorination especially in the process of electrolysis of seawater into chlorine. The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are.
 Source: tallbloke.wordpress.com
Source: tallbloke.wordpress.com
This process is called electrolysis. Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics. Common salt dissolved in water to form brine. When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. 2nacl 2 h 2 o 2naoh h 2 c l 2.
 Source: sciencedirect.com
Source: sciencedirect.com
Working in groups learners will. Salt electrochlorination is through electrolysis of salt water or sea water in the anode produced by the reaction of chlorine gas and water in the solution of hypochlorite with a certain degree of disinfection and sterilization effect. Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics. When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells.
 Source: mammothmemory.net
Source: mammothmemory.net
Common salt dissolved in water to form brine. The right hand side represents the products. Working in groups learners will. Naoh sodium hydroxide solution in the catholytic section the cathodic solution hydrogen gas which is given off at the cathode. This process is called electrolysis.
 Source: youtube.com
Source: youtube.com
Water splitting with electricity electrolysis is a simple and old idea. Salt electrochlorination is through electrolysis of salt water or sea water in the anode produced by the reaction of chlorine gas and water in the solution of hypochlorite with a certain degree of disinfection and sterilization effect. Chloride is negatively charged and is attracted to the positive side of the electrical charge where it bonds with oxygen and hydrogen from the water. This process is called electrolysis. Hydrogen gas will be seen to bubble up at the cathode and chlorine gas will bubble at the anode.
 Source: cascade.co.nz
Source: cascade.co.nz
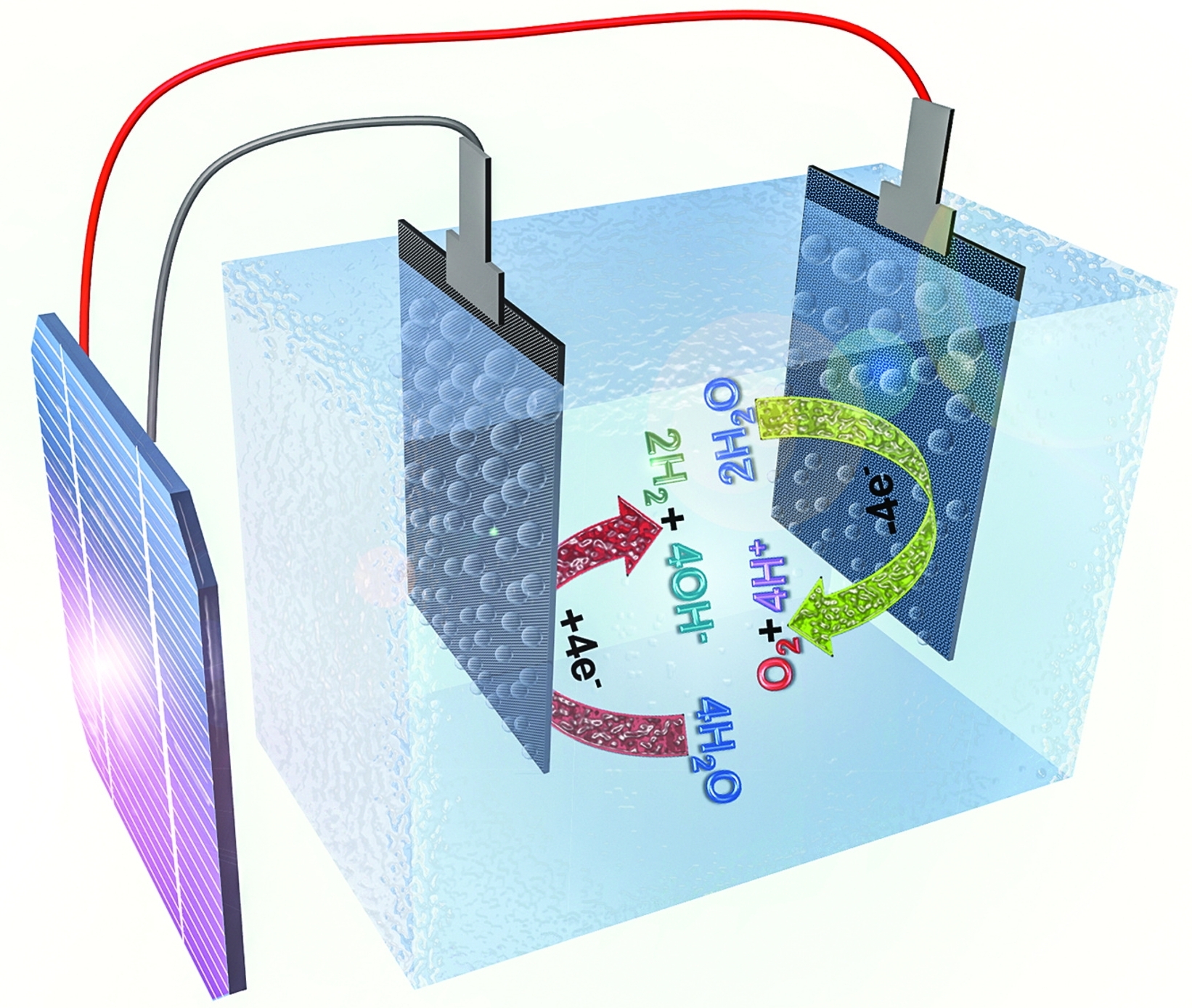
A power source connects to two electrodes placed in water. Two options exist for performance of this electrolysis. When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. A power source connects to two electrodes placed in water. The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are.
 Source: phys.org
Source: phys.org
Water splitting with electricity electrolysis is a simple and old idea. Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics. Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. Hydrogen gas will be seen to bubble up at the cathode and chlorine gas will bubble at the anode. 2nacl 2 h 2 o 2naoh h 2 c l 2.
 Source: blog.orendatech.com
Source: blog.orendatech.com
The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are. Common salt dissolved in water to form brine. This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells. Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. But if you try the same thing with saltwater the chloride ions in salt salt is a mix of chloride and sodium atoms will corrode the anode and.
 Source: cen.acs.org
Source: cen.acs.org
The first option is to subject the water to total desalinization to remove all impurities and produce essentially distilled water. This is a hands on lab activity about the chemical composition and conductivity of water. Negatively charged chloride in seawater salt can corrode the anode however limiting the system s lifespan. This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells. Two options exist for performance of this electrolysis.
 Source: revisionscience.com
Source: revisionscience.com
Salt water chlorination is a process that uses dissolved salt 1000 36 000 ppm or 1 36 g l for the chlorination of swimming pools and hot tubs. So far so good. But if you try the same thing with saltwater the chloride ions in salt salt is a mix of chloride and sodium atoms will corrode the anode and. In the process of salt electrochlorination especially in the process of electrolysis of seawater into chlorine. The chlorine generator also known as salt cell salt generator salt chlorinator or swg uses electrolysis in the presence of dissolved salt to produce chlorine gas or its dissolved forms hypochlorous acid and sodium hypochlorite which are.
 Source: daiso-eng.co.jp
Source: daiso-eng.co.jp
This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells. A power source connects to two electrodes placed in water. This distilled water can then be subjected to electrolysis in conventional alkaline electrolyte electrolysis cells. When an electrical charge is passed through a salt nacl solution the sodium separates from the chloride. Nacl aq can be reliably electrolysed to produce hydrogen.
 Source: sciencedirect.com
Source: sciencedirect.com
A power source connects to two electrodes placed in water. Chloride is negatively charged and is attracted to the positive side of the electrical charge where it bonds with oxygen and hydrogen from the water. Hydrogen gas will be seen to bubble up at the cathode and chlorine gas will bubble at the anode. Electrolysis of an aqueous solution of table salt nacl or sodium chloride produces aqueous sodium hydroxide and chlorine although usually only in minute amounts. Conduct an experiment involving the process of electrolysis prepare an experiment to better understand the process of ion exchange discuss and research the softness and hardness of water and use the periodic table to identify elements and learn their characteristics.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis of saltwater by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.