Electrolysis of copper sulfate solution
Electrolysis Of Copper Sulfate Solution. My intention for this observation is to find out how the current has an effect on the mass of copper deposited in the electrolysis of copper sulphate and copper ii plates. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. In this practical students carry out the electrolysis of copper ii sulfate solution.
 Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. In this practical students carry out the electrolysis of copper ii sulfate solution. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. This class experiment can be done by students working either in pairs or threes. Electricity is passed through solutions containing copper compounds such as copper ii sulfate.
Copper is purified by electrolysis.
The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The use of copper electrodes illustrates how copper is refined industrially. Using the simple apparatus right diagram and two copper electrodes the products of the electrolysis of copper sulfate solution are i a copper deposit on the negative cathode electrode and ii copper dissolves at the positive anode electrode. This class experiment can be done by students working either in pairs or threes. My intention for this observation is to find out how the current has an effect on the mass of copper deposited in the electrolysis of copper sulphate and copper ii plates. This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper.
 Source: youtube.com
Source: youtube.com
The anode positive electrode is made from impure copper and. As cuso 4 is an electrolyte it splits into cu cation and so 4 anion ions and move freely in the solution. Copper electrodes take part in the reactions and are described as non inert. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water.

The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. Copper is purified by electrolysis. As cuso 4 is an electrolyte it splits into cu cation and so 4 anion ions and move freely in the solution. The procedure is extremely simple just get a copper sulfate solution insert two electrodes and run a current through them. Aqueous copper ii sulfate about 0 5 m 200 cm 3.
 Source: docbrown.info
Source: docbrown.info
To investigate the electrolysis of copper sulfate solution using non inert electrodes. The anode positive electrode is made from impure copper and. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. Aqueous copper ii sulfate about 0 5 m 200 cm 3. In this practical students carry out the electrolysis of copper ii sulfate solution.
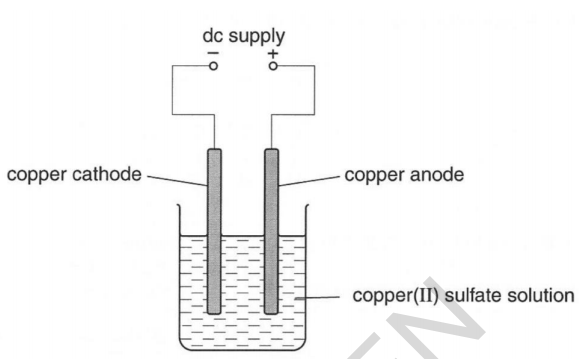 Source: elevise.co.uk
Source: elevise.co.uk
This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. For my gcse chemistry assessment i will be investigating the electrolysis of copper sulphate solution with the copper ii plates. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. The procedure is extremely simple just get a copper sulfate solution insert two electrodes and run a current through them.
 Source: docbrown.info
Source: docbrown.info
Electricity is passed through solutions containing copper compounds such as copper ii sulfate. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. This class experiment can be done by students working either in pairs or threes. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. Aqueous copper ii sulfate about 0 5 m 200 cm 3.
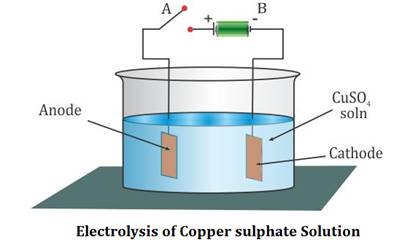 Source: topperlearning.com
Source: topperlearning.com
Copper is purified by electrolysis. At the positive electrode copper. The use of copper electrodes illustrates how copper is refined industrially. As cuso 4 is an electrolyte it splits into cu cation and so 4 anion ions and move freely in the solution. Aqueous copper ii sulfate about 0 5 m 200 cm 3.
 Source: electrical4u.com
Source: electrical4u.com
The products of electrolysing copper sulfate solution with copper electrodes are copper metal and copper ions because the copper anode dissolves. This class experiment can be done by students working either in pairs or threes. My intention for this observation is to find out how the current has an effect on the mass of copper deposited in the electrolysis of copper sulphate and copper ii plates. The products of electrolysing copper sulfate solution with copper electrodes are copper metal and copper ions because the copper anode dissolves. For my gcse chemistry assessment i will be investigating the electrolysis of copper sulphate solution with the copper ii plates.
 Source: researchgate.net
Source: researchgate.net
The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. This class experiment can be done by students working either in pairs or threes. Very few materials meet this condition platinum lead dioxide and carbon among them. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. To investigate the electrolysis of copper sulfate solution using non inert electrodes.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
The products of electrolysing copper sulfate solution with copper electrodes are copper metal and copper ions because the copper anode dissolves. My intention for this observation is to find out how the current has an effect on the mass of copper deposited in the electrolysis of copper sulphate and copper ii plates. This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. Using the simple apparatus right diagram and two copper electrodes the products of the electrolysis of copper sulfate solution are i a copper deposit on the negative cathode electrode and ii copper dissolves at the positive anode electrode.
 Source: m.youtube.com
Source: m.youtube.com
Electrolysis of copper sulfate. Electrolysis of copper sulfate. Very few materials meet this condition platinum lead dioxide and carbon among them. This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. The procedure is extremely simple just get a copper sulfate solution insert two electrodes and run a current through them.

Very few materials meet this condition platinum lead dioxide and carbon among them. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. Copper electrodes take part in the reactions and are described as non inert. The use of copper electrodes illustrates how copper is refined industrially. My intention for this observation is to find out how the current has an effect on the mass of copper deposited in the electrolysis of copper sulphate and copper ii plates.
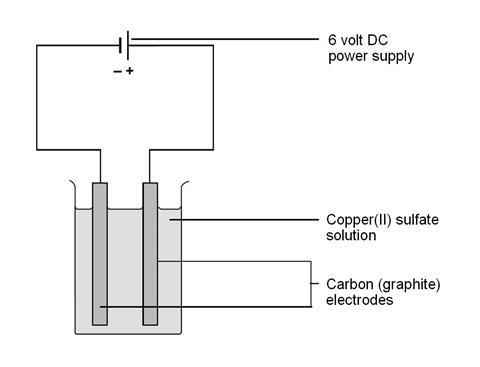 Source: edu.rsc.org
Source: edu.rsc.org
The use of copper electrodes illustrates how copper is refined industrially. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. Very few materials meet this condition platinum lead dioxide and carbon among them. Electrolysis of copper sulfate. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions.
 Source: docbrown.info
Source: docbrown.info
Copper electrodes take part in the reactions and are described as non inert. This class experiment can be done by students working either in pairs or threes. To investigate the electrolysis of copper sulfate solution using non inert electrodes. For my gcse chemistry assessment i will be investigating the electrolysis of copper sulphate solution with the copper ii plates. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes.
 Source: docbrown.info
Source: docbrown.info
Very few materials meet this condition platinum lead dioxide and carbon among them. To investigate the electrolysis of copper sulfate solution using non inert electrodes. At the positive electrode copper. This experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. Using the simple apparatus right diagram and two copper electrodes the products of the electrolysis of copper sulfate solution are i a copper deposit on the negative cathode electrode and ii copper dissolves at the positive anode electrode.
 Source: pinterest.com
Source: pinterest.com
Electricity is passed through solutions containing copper compounds such as copper ii sulfate. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. At the positive electrode copper. Using the simple apparatus right diagram and two copper electrodes the products of the electrolysis of copper sulfate solution are i a copper deposit on the negative cathode electrode and ii copper dissolves at the positive anode electrode.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electrolysis of copper sulfate solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





