Electrolysis hydrogen fuel cell
Electrolysis Hydrogen Fuel Cell. You must regulate the temperature of a hydrogen fuel cell to maximize its use. Electrolysis is the process of using electricity to split water into its component parts of hydrogen and oxygen. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving.
 Principles Of The Electrolysis Of Water And The Hydrogen Fuel Cell Download Scientific Diagram From researchgate.net
Principles Of The Electrolysis Of Water And The Hydrogen Fuel Cell Download Scientific Diagram From researchgate.net
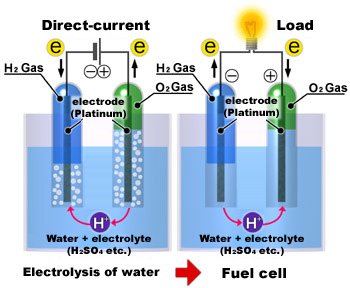
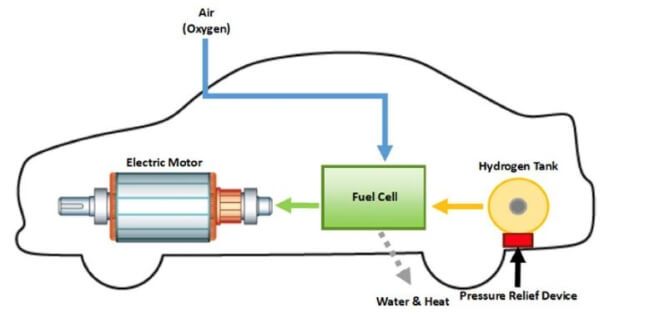
Hydrogen fuel cell hydrogen and oxygen can be combined in a fuel cell to produce electrical energy. A power supply passes a current through the cell splitting water into hydrogen and oxygen. This energy is directed into the electric motor and or the battery as needed. When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell. Electrolysis is the process of using electricity to split water into hydrogen and oxygen.
That hydrogen can then be stored for later use as fuel in a fuel cell vehicle to power a stationary fuel cell system or for power to gas applications.
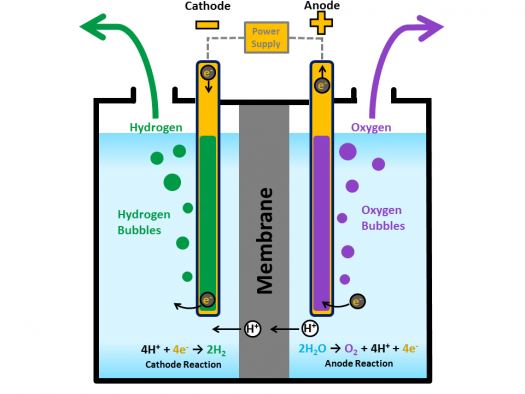
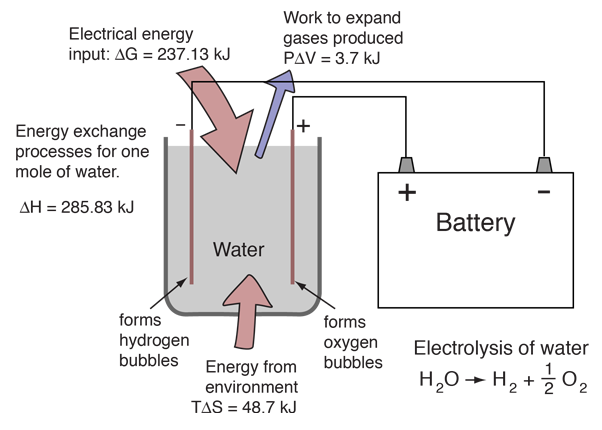
2 h20 l h2 g o2 g δhrxn 286 kj mol h2 each electrolyser cell consists of an anode and a cathode separated by a membrane submerged in water. Electrolysis is a promising option for hydrogen production from renewable resources. A fuel cell uses a chemical reaction to provide an external voltage as does a battery but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas. When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. This energy is directed into the electric motor and or the battery as needed. In a fuel cell hydrogen and oxygen are supplied to the anode and the cathode and they combine to form water while creating an electrical current that can be put to use.
 Source: fccj.jp
Source: fccj.jp
With commercial hydrogen fuel cell cars the operation of hydrogen electrolysis is some what opposite of an electrolyzer. Characterization testing addresses the operation of hydrogen producing stacks at variable current without a fixed power supply. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell. Hydrogen fuel cell hydrogen and oxygen can be combined in a fuel cell to produce electrical energy. That hydrogen can then be stored for later use as fuel in a fuel cell vehicle to power a stationary fuel cell system or for power to gas applications.
 Source: energy.gov
Source: energy.gov
In the fuel cell of an fcev hydrogen and oxygen generate electrical energy. If you want to operate a hydrogen fuel cell at its rate of highest efficiency then you must maintain temperature conditions below 212 f at all times. Characteristics units 2011 status 2015 target 2020 target. A power supply passes a current through the cell splitting water into hydrogen and oxygen. Distributed forecourt water electrolysis hydrogen production a b c.
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
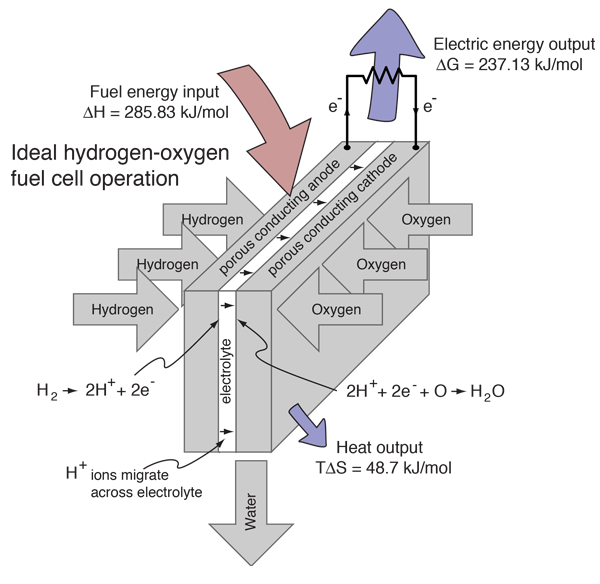
This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell. You must regulate the temperature of a hydrogen fuel cell to maximize its use. This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. A fuel cell uses a chemical reaction to provide an external voltage as does a battery but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas.
 Source: electropages.com
Source: electropages.com
This energy is directed into the electric motor and or the battery as needed. When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. With commercial hydrogen fuel cell cars the operation of hydrogen electrolysis is some what opposite of an electrolyzer. This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. This reaction takes place in a unit called an electrolyzer.
 Source: nrel.gov
Source: nrel.gov
Electrolysis is a promising option for hydrogen production from renewable resources. Electrolysis is a promising option for hydrogen production from renewable resources. 2 h20 l h2 g o2 g δhrxn 286 kj mol h2 each electrolyser cell consists of an anode and a cathode separated by a membrane submerged in water. More information about targets can be found in the hydrogen production section of the fuel cell technologies office s multi year research development and demonstration plan. If you want to operate a hydrogen fuel cell at its rate of highest efficiency then you must maintain temperature conditions below 212 f at all times.
 Source: fchea.org
Source: fchea.org
You must regulate the temperature of a hydrogen fuel cell to maximize its use. With commercial hydrogen fuel cell cars the operation of hydrogen electrolysis is some what opposite of an electrolyzer. In commercially available electrolyzer systems the electrolyzer stack accepts dc power input from its onboard power converter. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. A fuel cell uses a chemical reaction to provide an external voltage as does a battery but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas.
 Source: butane.chem.uiuc.edu
Source: butane.chem.uiuc.edu
When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. That hydrogen can then be stored for later use as fuel in a fuel cell vehicle to power a stationary fuel cell system or for power to gas applications. With commercial hydrogen fuel cell cars the operation of hydrogen electrolysis is some what opposite of an electrolyzer. A power supply passes a current through the cell splitting water into hydrogen and oxygen. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell.
 Source: researchgate.net
Source: researchgate.net
Electrolysis is the process of using electricity to split water into its component parts of hydrogen and oxygen. Hydrogen fuel cell hydrogen and oxygen can be combined in a fuel cell to produce electrical energy. The electrolyzer regulates power to the stack and operates at a fixed stack current. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell. In the fuel cell of an fcev hydrogen and oxygen generate electrical energy.
 Source: power-technology.com
Source: power-technology.com
In the fuel cell of an fcev hydrogen and oxygen generate electrical energy. 2 h20 l h2 g o2 g δhrxn 286 kj mol h2 each electrolyser cell consists of an anode and a cathode separated by a membrane submerged in water. Electrolysis is a promising option for hydrogen production from renewable resources. The electrolyzer regulates power to the stack and operates at a fixed stack current. Hydrogen fuel cell hydrogen and oxygen can be combined in a fuel cell to produce electrical energy.
 Source: americanhistory.si.edu
Source: americanhistory.si.edu
When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. The electrolyser itself is a series of cells with an electrolysis reaction taking place. This energy is directed into the electric motor and or the battery as needed. Electrolysis is a promising option for hydrogen production from renewable resources. In fuel cell technology a process known as reverse electrolysis takes place in which hydrogen reacts with oxygen in the fuel cell.
 Source: hydrogeneurope.eu
Source: hydrogeneurope.eu
Characterization testing addresses the operation of hydrogen producing stacks at variable current without a fixed power supply. A power supply passes a current through the cell splitting water into hydrogen and oxygen. You must regulate the temperature of a hydrogen fuel cell to maximize its use. The electrolyzer regulates power to the stack and operates at a fixed stack current. Distributed forecourt water electrolysis hydrogen production a b c.
 Source: researchgate.net
Source: researchgate.net
The electrolyzer regulates power to the stack and operates at a fixed stack current. A fuel cell uses a chemical reaction to provide an external voltage as does a battery but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas. Electrolysis is the process of using electricity to split water into its component parts of hydrogen and oxygen. When temperatures rise above this level then you will not receive the same levels of fuel efficiency when driving. Characterization testing addresses the operation of hydrogen producing stacks at variable current without a fixed power supply.
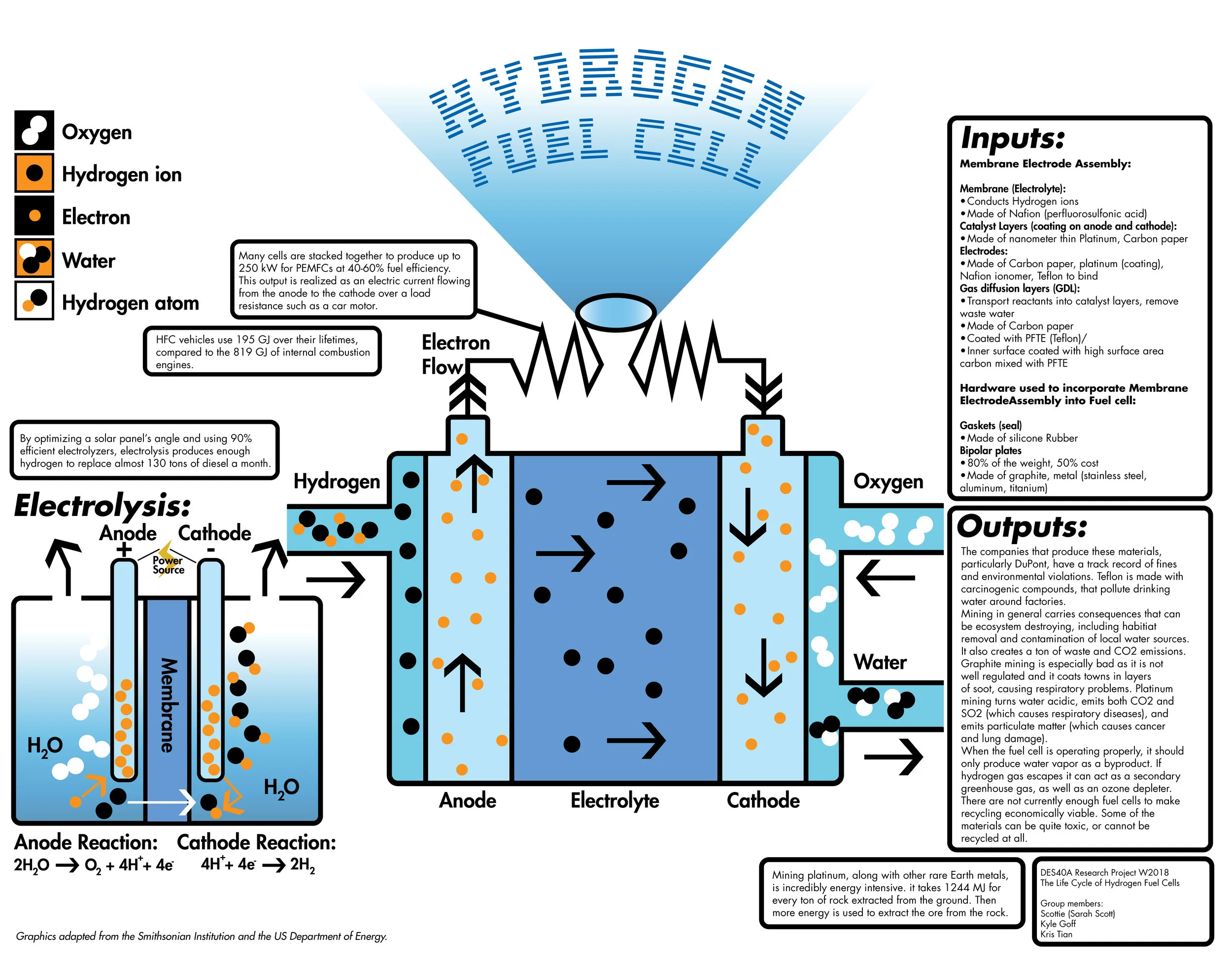
2 h20 l h2 g o2 g δhrxn 286 kj mol h2 each electrolyser cell consists of an anode and a cathode separated by a membrane submerged in water. Characteristics units 2011 status 2015 target 2020 target. This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. In the fuel cell of an fcev hydrogen and oxygen generate electrical energy. Electrolysis is the process of using electricity to split water into its component parts of hydrogen and oxygen.
 Source: intelligent-energy.com
Source: intelligent-energy.com
This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. The electrolyser itself is a series of cells with an electrolysis reaction taking place. This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. Electrolysis is the process of using electricity to split water into hydrogen and oxygen. Electrolysis is a promising option for hydrogen production from renewable resources.
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
A power supply passes a current through the cell splitting water into hydrogen and oxygen. A fuel cell uses a chemical reaction to provide an external voltage as does a battery but differs from a battery in that the fuel is continually supplied in the form of hydrogen and oxygen gas. This process can also be reversed to generate electricity when hydrogen and oxygen gases interact in a fuel cell nasa has used fuel cells to power satellites and space capsules since the 1960s. In a fuel cell hydrogen and oxygen are supplied to the anode and the cathode and they combine to form water while creating an electrical current that can be put to use. Characterization testing addresses the operation of hydrogen producing stacks at variable current without a fixed power supply.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electrolysis hydrogen fuel cell by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.