Electrolysis copper plating
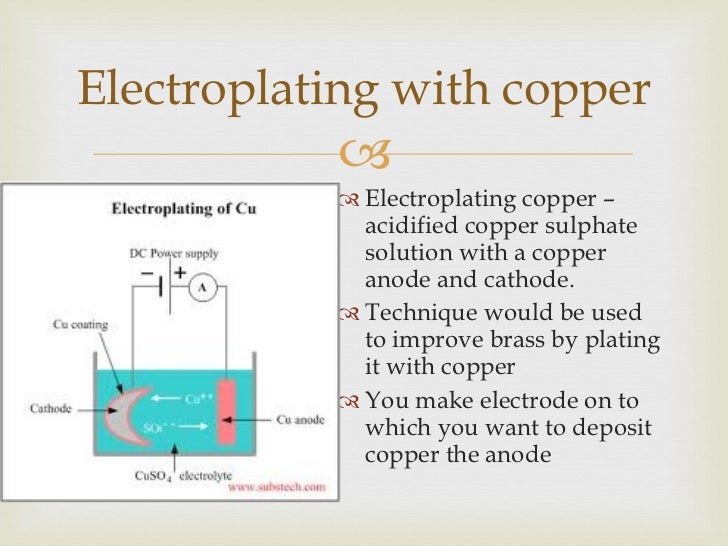
Electrolysis Copper Plating. The copper rod is the anode and the item is the cathod. Into a container of water are placed a copper rod and the item. Like with silver plating copper deposition can be used to purify copper. When we switch on the power the copper sulfate solution splits into ions.
 Detailed Procedures Of Plating Copper For Pcb Processing Pcb Manufacturing Information Pcbway From pcbway.com
Detailed Procedures Of Plating Copper For Pcb Processing Pcb Manufacturing Information Pcbway From pcbway.com
Plating copper on copper demonstration. I copper electroplating copper plating by electrolysis of a copper salt solution ve cathode cu 2 aq 2e cu s electron gain reduction copper deposited electroplated on the cathode object dull object might look a lot prettier. Prepare the key for copper plating by cleaning it with a thin layer of. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it. The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item.
The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item.
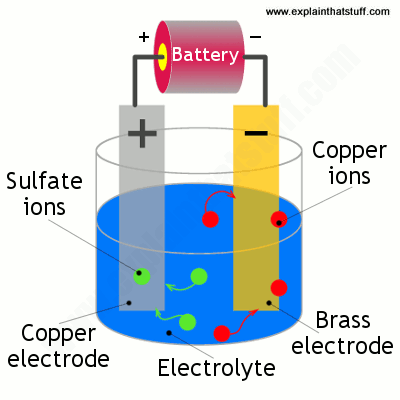
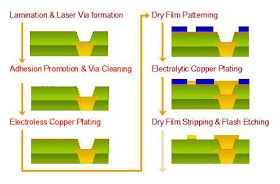
Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate like metal or plastic the process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde. If we re copper plating some brass we need a copper electrode a brass electrode and a solution of a copper based compound such as copper sulfate solution. Ve anode cu s 2e cu 2 aq. Like with silver plating copper deposition can be used to purify copper. In this case both the electrodes are made from copper. Table 17 8 sample data and results of calculations of a copper zinc electrolysis cell.
 Source: slideshare.net
Source: slideshare.net
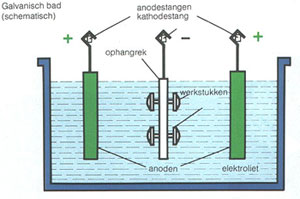
The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it. The copper rod is the anode and the item is the cathod. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current.
 Source: uk-finishing.org.uk
Source: uk-finishing.org.uk
Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate like metal or plastic the process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde. I copper electroplating copper plating by electrolysis of a copper salt solution ve cathode cu 2 aq 2e cu s electron gain reduction copper deposited electroplated on the cathode object dull object might look a lot prettier. Using electricity you can coat the metal of one electrode with the metal of the other with an electroplating process also known as electrochemistry. This demonstration is an application of faraday s law. A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it.
This demonstration is an application of faraday s law. Plating copper on copper demonstration. If we re copper plating some brass we need a copper electrode a brass electrode and a solution of a copper based compound such as copper sulfate solution. Table 17 8 sample data and results of calculations of a copper zinc electrolysis cell. This demonstration is an application of faraday s law.
 Source: explainthatstuff.com
Source: explainthatstuff.com
When we switch on the power the copper sulfate solution splits into ions. The copper rod is the anode and the item is the cathod. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. The top of one cu electrode is connected to the negative terminal of a d c. Prepare the key for copper plating by cleaning it with a thin layer of.
 Source: pcbway.com
Source: pcbway.com
This demonstration is an application of faraday s law. Ve anode cu s 2e cu 2 aq. The top of one cu electrode is connected to the negative terminal of a d c. Into a container of water are placed a copper rod and the item. This demonstration is an application of faraday s law.
 Source: docbrown.info
Source: docbrown.info
Table 17 8 sample data and results of calculations of a copper zinc electrolysis cell. In this case both the electrodes are made from copper. Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate like metal or plastic the process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde. Like with silver plating copper deposition can be used to purify copper. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item.
 Source: docbrown.info
Source: docbrown.info
A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it. Plating copper on copper demonstration. Prepare the key for copper plating by cleaning it with a thin layer of. A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it. Unlike electroplating electroless plating processes in general not require passing an electric current through the.
 Source: docbrown.info
Source: docbrown.info
This demonstration is an application of faraday s law. A postage balance is used to measure the mass of two copper electrodes prior to the demonstration. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current. Ve anode cu s 2e cu 2 aq. When we switch on the power the copper sulfate solution splits into ions.
 Source: docbrown.info
Source: docbrown.info
When we switch on the power the copper sulfate solution splits into ions. Table 17 8 sample data and results of calculations of a copper zinc electrolysis cell. The object that is to be plated is connected to the negative terminal of the power supply. Copper plating is the process of plating a layer of copper electrolytically on the surface of an item. When we switch on the power the copper sulfate solution splits into ions.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
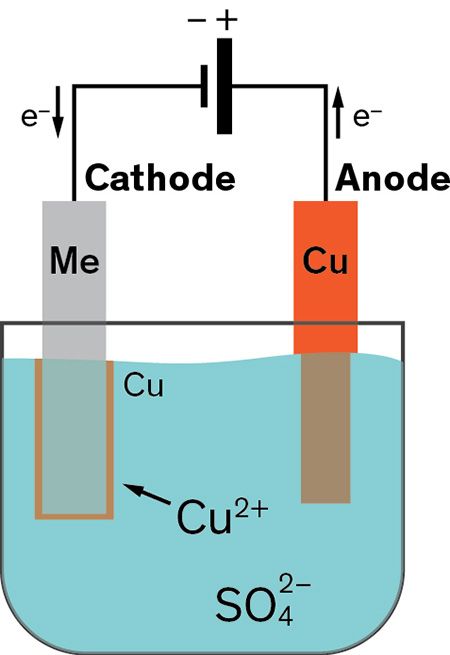
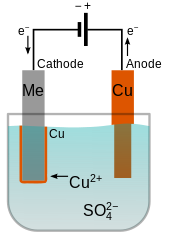
Into a container of water are placed a copper rod and the item. Electroless copper plating is a chemical process that deposits an even layer of copper on the surface of a solid substrate like metal or plastic the process involves dipping the substrate in a water solution containing copper salts and a reducing agent such as formaldehyde. The copper rod is the anode and the item is the cathod. The electrolyte is a copper sulphate solution. The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
Copper ions which are positively charged are attracted to the negatively charged brass electrode and slowly. The top of one cu electrode is connected to the negative terminal of a d c. Copper ions which are positively charged are attracted to the negatively charged brass electrode and slowly. Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current. A white coating appears on the copper electrode almost immediately and after a few minutes the copper electrode has a definite zinc plating on it.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The top of one cu electrode is connected to the negative terminal of a d c.
 Source: byjus.com
Source: byjus.com
The top of one cu electrode is connected to the negative terminal of a d c. This demonstration is an application of faraday s law. Prepare the key for copper plating by cleaning it with a thin layer of. The copper rod is the anode and the item is the cathod. Plating copper on copper demonstration.
 Source: pcbfab1001.blogspot.com
Source: pcbfab1001.blogspot.com
Electroplating uses a form of electrolysis in which the electrodes play a bigger role than just conducting the current. The water contains an ionic solution which allows a direct electric current to flow from the copper rod to the item. It takes place in an electrolytic cell where electrolysis which uses direct electric current to dissolve a copper rod and transport the copper ions to the item. In this case both the electrodes are made from copper. Unlike electroplating electroless plating processes in general not require passing an electric current through the.
 Source: researchgate.net
Source: researchgate.net
The object that is to be plated is connected to the negative terminal of the power supply. A piece of copper is connected to the positive terminal. Ve anode cu s 2e cu 2 aq. Like with silver plating copper deposition can be used to purify copper. I copper electroplating copper plating by electrolysis of a copper salt solution ve cathode cu 2 aq 2e cu s electron gain reduction copper deposited electroplated on the cathode object dull object might look a lot prettier.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electrolysis copper plating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.