Electrodes for electrolysis
Electrodes For Electrolysis. In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads. A porous graphite plate is coated on one face with a layer of tio 2 doped with a mixture of ruo 2 and iro 2. For example water electrolysis is an accepted method for hypochloride generation. Carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free electrons they have available for transfer.
What Happens If I Use A Graphite Electrode In Water Electrolysis Quora From quora.com
This means it can be used to facilitate a wide range of different reactions. A bipolar electrode for electrolysis of water. The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive. Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates. The coatings are applied as polysilicate based paints contain ing particles of the desired metals. A porous graphite plate is coated on one face with a layer of tio 2 doped with a mixture of ruo 2 and iro 2.
The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive.
The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive. This means it can be used to facilitate a wide range of different reactions. The invention relates to an electrode for use in alkaline water electrolysis at least consisting of a support a deposited on the carrier gas and liquid tight corrosion protection layer and deposited on the corrosion protection layer porous metal layer of iron or nickel wherein the pores of the porous metal layer with increasing distance from the anticorrosion layer have a gradually. For example water electrolysis is an accepted method for hypochloride generation. Platinum or carbon electrodes are examples of inert electrodes. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses.
 Source: ibchem.com
Source: ibchem.com
Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates. The uncoated surface of the porous graphite plate may be grooved. Not only is carbon an efficient conductor it also has a very high melting point. A bipolar electrode for electrolysis of water. The discharge of ions during electrolysis can be influenced by the types of electrodes used.
 Source: scielo.br
Source: scielo.br
The uncoated surface of the porous graphite plate may be grooved. Some electrodes are inert do not take part in electrolytic reaction while other electrodes are reactive which may influence the ionic discharge. Not only is carbon an efficient conductor it also has a very high melting point. For example water electrolysis is an accepted method for hypochloride generation. Electrolysis in simple electric cell.
 Source: toppr.com
Source: toppr.com
In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads. Carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free electrons they have available for transfer. Some electrodes are inert do not take part in electrolytic reaction while other electrodes are reactive which may influence the ionic discharge. A bipolar electrode for electrolysis of water. Platinum or carbon electrodes are examples of inert electrodes.
 Source: docbrown.info
Source: docbrown.info
The enclosure has therein a plurality of compartments having open sides through one wall of the enclosure. Platinum or carbon electrodes are examples of inert electrodes. In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads. Not only is carbon an efficient conductor it also has a very high melting point. Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates.
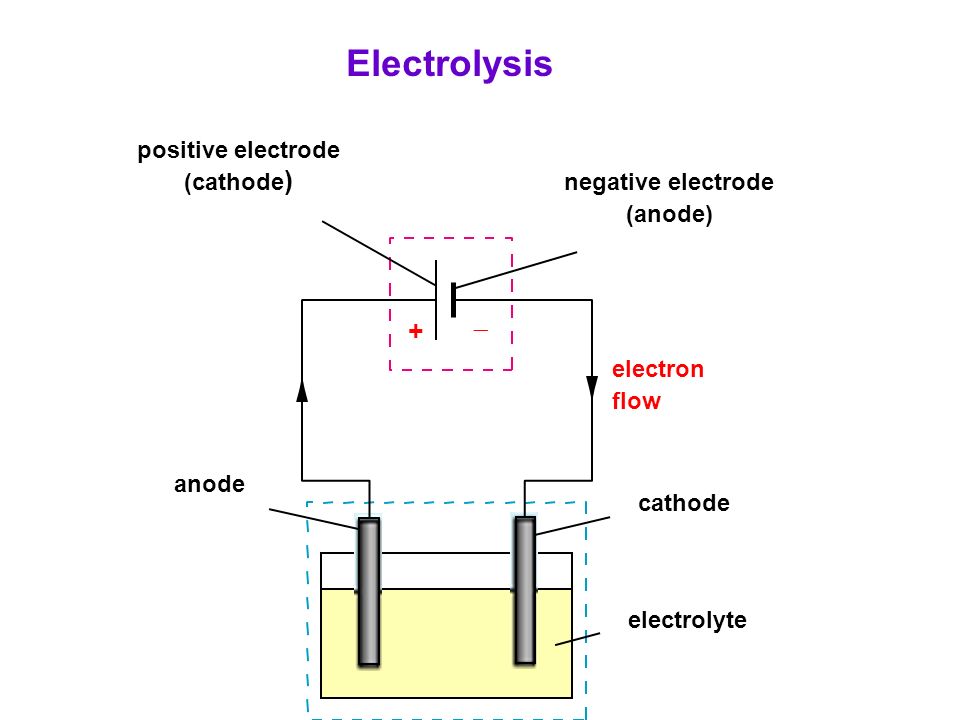 Source: slideplayer.com
Source: slideplayer.com
The enclosure has therein a plurality of compartments having open sides through one wall of the enclosure. The uncoated surface of the porous graphite plate may be grooved. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. The coating is sintered into a porous. Electrolysis in simple electric cell.
Source: quora.com
This means it can be used to facilitate a wide range of different reactions. The discharge of ions during electrolysis can be influenced by the types of electrodes used. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. A bipolar electrode for electrolysis of water. In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads.
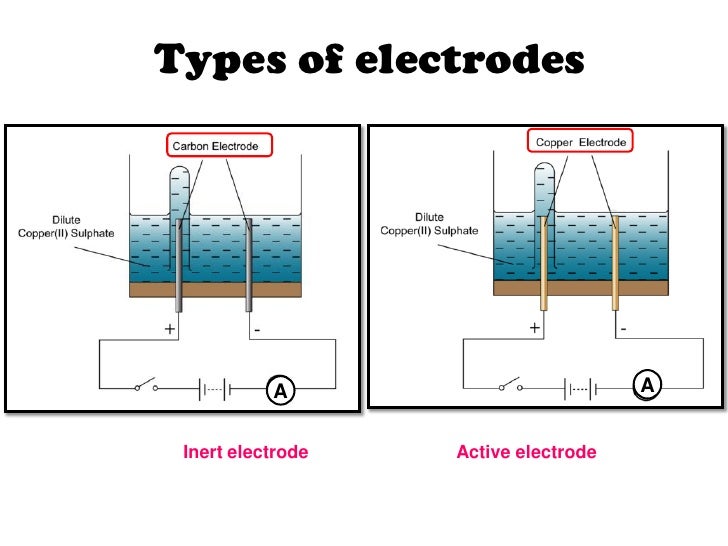 Source: slideshare.net
Source: slideshare.net
Carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free electrons they have available for transfer. This means it can be used to facilitate a wide range of different reactions. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads. Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates.
 Source: s-cool.co.uk
Source: s-cool.co.uk
The coating is sintered into a porous. The enclosure has therein a plurality of compartments having open sides through one wall of the enclosure. Not only is carbon an efficient conductor it also has a very high melting point. Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates. The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive.
 Source: lookgoodandstudyhard.blogspot.com
Source: lookgoodandstudyhard.blogspot.com
The discharge of ions during electrolysis can be influenced by the types of electrodes used. The invention relates to an electrode for use in alkaline water electrolysis at least consisting of a support a deposited on the carrier gas and liquid tight corrosion protection layer and deposited on the corrosion protection layer porous metal layer of iron or nickel wherein the pores of the porous metal layer with increasing distance from the anticorrosion layer have a gradually. Not only is carbon an efficient conductor it also has a very high melting point. Some electrodes are inert do not take part in electrolytic reaction while other electrodes are reactive which may influence the ionic discharge. The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
In a bipolar electrode electrolysis apparatus particularly for the electrolysis of saline solutions an enclosure is formed of electrically insulating material and has monopolar terminal electrodes for connection to current leads. Platinum or carbon electrodes are examples of inert electrodes. Electrolysis in simple electric cell. Carbon electrodes are used in electrolysis due to their competence as a conductor and the number of free electrons they have available for transfer. The coatings are applied as polysilicate based paints contain ing particles of the desired metals.

The coatings are applied as polysilicate based paints contain ing particles of the desired metals. The nickel foam electrode was best at letting the bubbles escape but it had a significantly lower surface area than the other two electrodes making it less productive. A bipolar electrode for electrolysis of water. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. The discharge of ions during electrolysis can be influenced by the types of electrodes used.

The discharge of ions during electrolysis can be influenced by the types of electrodes used. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. Electrodes for alkaline water electrolysis have been made by applying high specific surface area coatings of nickel or nickel iron alloy to steel or nickel substrates. The enclosure has therein a plurality of compartments having open sides through one wall of the enclosure. Platinum or carbon electrodes are examples of inert electrodes.
 Source: researchgate.net
Source: researchgate.net
Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. Platinum or carbon electrodes are examples of inert electrodes. A bipolar electrode for electrolysis of water. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. This means it can be used to facilitate a wide range of different reactions.
 Source: yischemistry.pbworks.com
Source: yischemistry.pbworks.com
This means it can be used to facilitate a wide range of different reactions. The discharge of ions during electrolysis can be influenced by the types of electrodes used. Some electrodes are inert do not take part in electrolytic reaction while other electrodes are reactive which may influence the ionic discharge. The invention relates to an electrode for use in alkaline water electrolysis at least consisting of a support a deposited on the carrier gas and liquid tight corrosion protection layer and deposited on the corrosion protection layer porous metal layer of iron or nickel wherein the pores of the porous metal layer with increasing distance from the anticorrosion layer have a gradually. This means it can be used to facilitate a wide range of different reactions.
 Source: docbrown.info
Source: docbrown.info
The coatings are applied as polysilicate based paints contain ing particles of the desired metals. The coatings are applied as polysilicate based paints contain ing particles of the desired metals. The coating is sintered into a porous. Electrodes for water electrolysis used for hypochloride generation and sterilization of water anode and cathode electrodes for water electrolysis are widely used for various electrolysis requirements such as hygiene food processing and other home uses. A bipolar electrode for electrolysis of water.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electrodes for electrolysis by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






