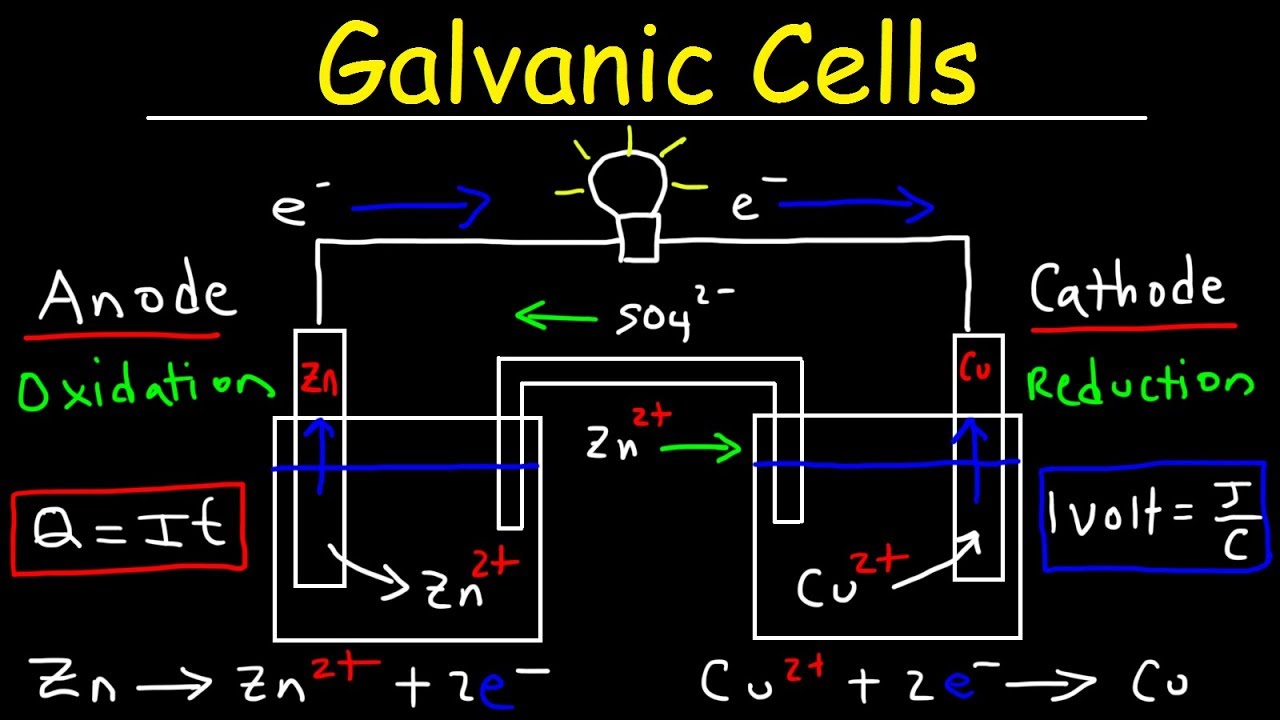
Electricity from salt water
Electricity From Salt Water. The electrolysis method was used to produce the electricity from saltwater. Saltwater can serve as the electrolyte in a battery generating electricity. Distilled water is pure and free of salts. A battery has three parts.
 Generate Electricity From Salt Water Schoolproject In From schoolproject.in
Generate Electricity From Salt Water Schoolproject In From schoolproject.in
Water is comprised of two elements hydrogen and oxygen. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. These ions are what carry electricity through water with an electric current. A battery has three parts. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. In short salt water can help to produce electricity.
While this can be done on a large scale let s try a small scale science project to see how it works.
When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. An electrolyte and two electrodes which are made of different materials often metals. These ions are what carry electricity through water with an electric current. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. A battery has three parts.
 Source: freescienceproject.com
Source: freescienceproject.com
A battery has three parts. Thus it is a very poor conductor of electricity. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. These ions are what carry electricity through water with an electric current. A battery has three parts.
 Source: scienceproject.com
Source: scienceproject.com
In short salt water can help to produce electricity. In short salt water can help to produce electricity. While this can be done on a large scale let s try a small scale science project to see how it works. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. Saltwater can serve as the electrolyte in a battery generating electricity.
 Source: youtube.com
Source: youtube.com
Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. Distilled water is pure and free of salts. A battery has three parts. An electrolyte and two electrodes which are made of different materials often metals. Thus it is a very poor conductor of electricity.
 Source: teachengineering.org
Source: teachengineering.org
Water is comprised of two elements hydrogen and oxygen. Thus it is a very poor conductor of electricity. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. Saltwater can serve as the electrolyte in a battery generating electricity.
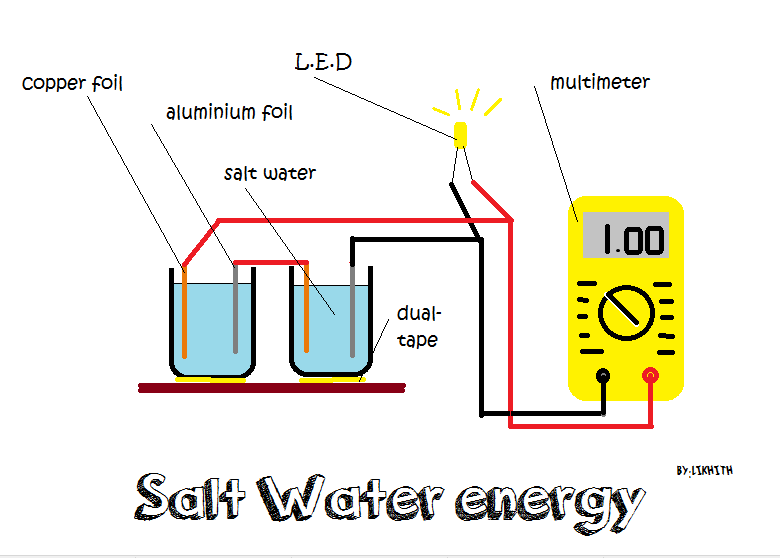 Source: instructables.com
Source: instructables.com
When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. These ions are what carry electricity through water with an electric current. Saltwater can serve as the electrolyte in a battery generating electricity. A battery has three parts.
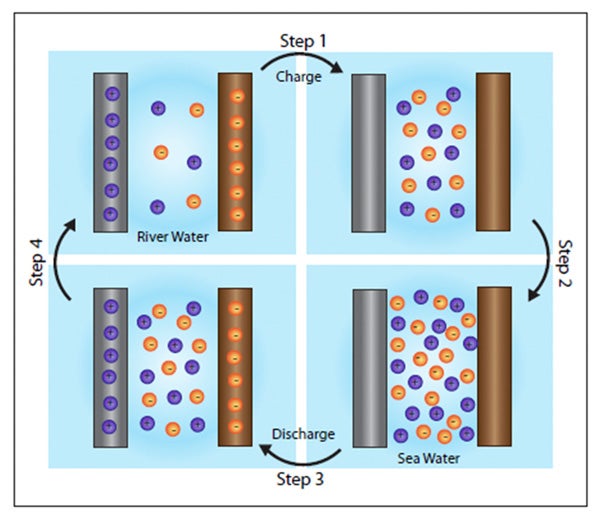 Source: news.stanford.edu
Source: news.stanford.edu
An electrolyte and two electrodes which are made of different materials often metals. Thus it is a very poor conductor of electricity. While this can be done on a large scale let s try a small scale science project to see how it works. In short salt water can help to produce electricity. A battery has three parts.
 Source: youtube.com
Source: youtube.com
The electrolysis method was used to produce the electricity from saltwater. Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. In short salt water can help to produce electricity. An electrolyte and two electrodes which are made of different materials often metals. A battery has three parts.
 Source: schoolproject.in
Source: schoolproject.in
These ions are what carry electricity through water with an electric current. While this can be done on a large scale let s try a small scale science project to see how it works. A battery has three parts. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. These ions are what carry electricity through water with an electric current.
 Source: pinterest.com
Source: pinterest.com
Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity. These ions are what carry electricity through water with an electric current. The electrolysis method was used to produce the electricity from saltwater. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity.
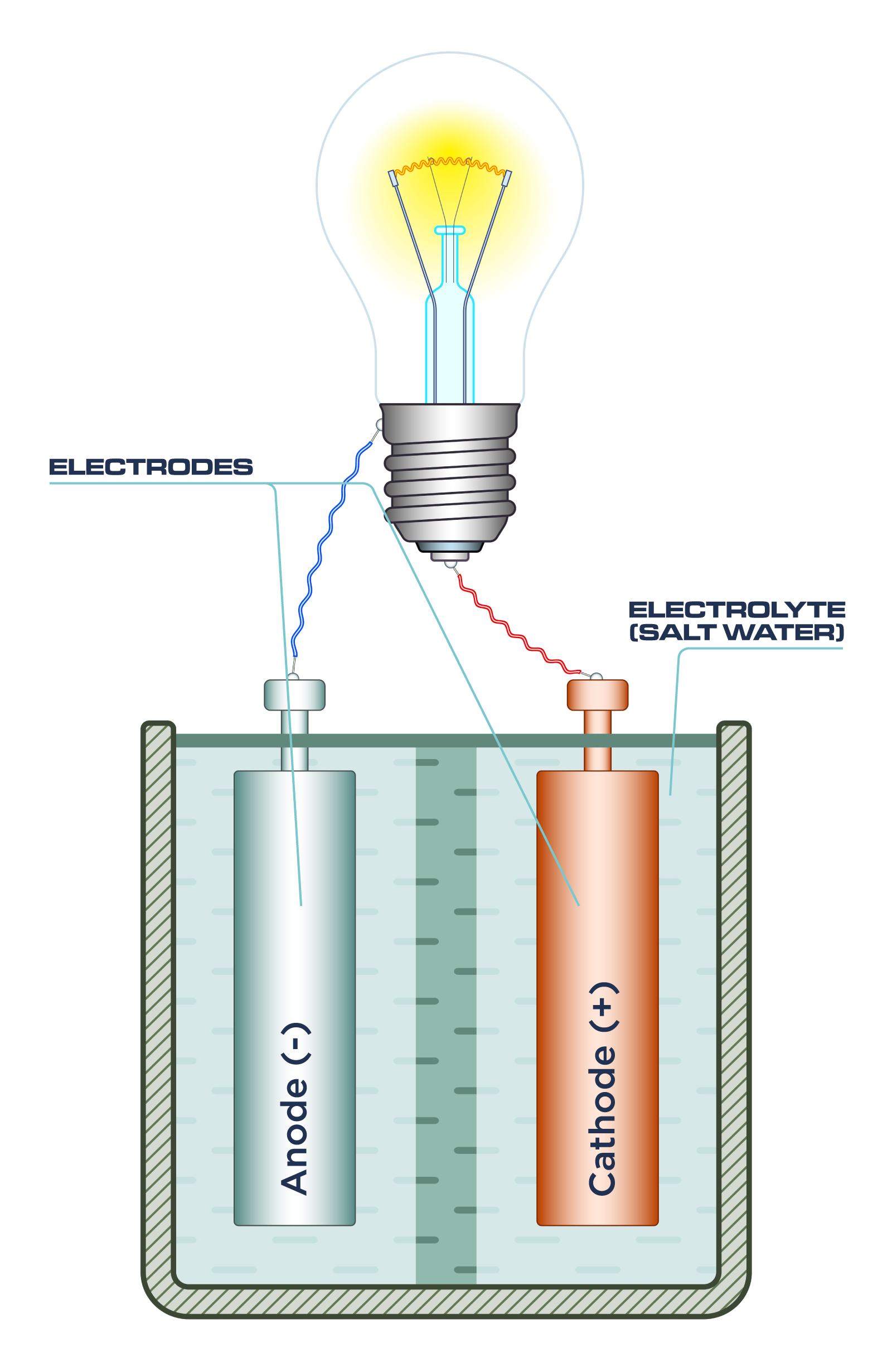 Source: ourfuture.energy
Source: ourfuture.energy
By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. An electrolyte and two electrodes which are made of different materials often metals. While this can be done on a large scale let s try a small scale science project to see how it works. A battery has three parts. Thus it is a very poor conductor of electricity.
 Source: pinterest.com
Source: pinterest.com
The electrolysis method was used to produce the electricity from saltwater. Saltwater can serve as the electrolyte in a battery generating electricity. Water is comprised of two elements hydrogen and oxygen. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. These ions are what carry electricity through water with an electric current.
 Source: aliexpress.com
Source: aliexpress.com
Saltwater can serve as the electrolyte in a battery generating electricity. In short salt water can help to produce electricity. These ions are what carry electricity through water with an electric current. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. Thus it is a very poor conductor of electricity.
 Source: in.pinterest.com
Source: in.pinterest.com
These ions are what carry electricity through water with an electric current. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. While this can be done on a large scale let s try a small scale science project to see how it works. A battery has three parts. Some of the first batteries made by alessandro volta around 1880 used saltwater silver and zinc to generate electricity.
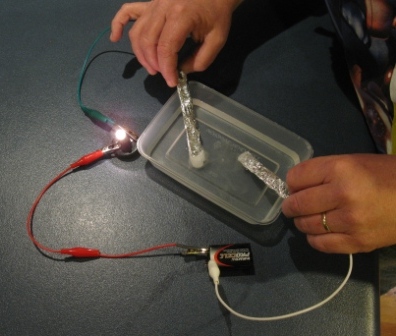
Saltwater can serve as the electrolyte in a battery generating electricity. These ions are what carry electricity through water with an electric current. The electrolysis method was used to produce the electricity from saltwater. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. While this can be done on a large scale let s try a small scale science project to see how it works.
 Source: m.youtube.com
Source: m.youtube.com
Saltwater can serve as the electrolyte in a battery generating electricity. An electrolyte and two electrodes which are made of different materials often metals. By adding ordinary table salt to distilled water it becomes an electrolyte solution that can conduct electricity. Saltwater can serve as the electrolyte in a battery generating electricity. In short salt water can help to produce electricity.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title electricity from salt water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.