Does water conduct electricity
Does Water Conduct Electricity. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. Completely pure water is actually an insulator and cannot conduct electricity. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Electricity seeks out oppositely charged particles to travel through.
 After Centuries Scientists Have Finally Figured Out How Water Conducts Electricity From sciencealert.com
After Centuries Scientists Have Finally Figured Out How Water Conducts Electricity From sciencealert.com
Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions. Pure water does not conduct electricity. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Any impurities like salts in the water enable it to conduct electricity. We re always told and taught that water conducts electricity.
Completely pure water is actually an insulator and cannot conduct electricity.
Electricity seeks out oppositely charged particles to travel through. We re always told and taught that water conducts electricity. By itself it is a poor conductor of electricity. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. Electricity seeks out oppositely charged particles to travel through.
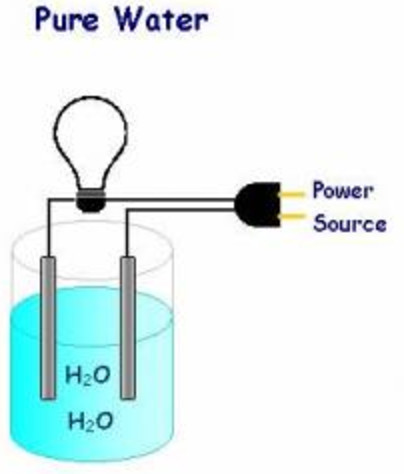 Source: classnotes.org.in
Source: classnotes.org.in
The short answer is. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. By itself it is a poor conductor of electricity. No pure water doesn t conduct electricity. Electricity seeks out oppositely charged particles to travel through.
 Source: howitworksdaily.com
Source: howitworksdaily.com
Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. The short answer is. Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions.
 Source: pinterest.com
Source: pinterest.com
When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Completely pure water is actually an insulator and cannot conduct electricity. When salts are dissolved in water they separate into different electrically charged atoms called ions. The short answer is. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair.
 Source: rookieparenting.com
Source: rookieparenting.com
We re always told and taught that water conducts electricity. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. The short answer is. When salts are dissolved in water they separate into different electrically charged atoms called ions. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair.
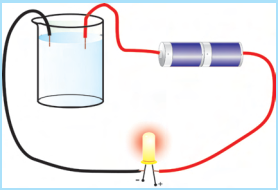 Source: toppr.com
Source: toppr.com
However water contains charged ions and impurities that make it a very good conductor of electricity. No pure water doesn t conduct electricity. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Pure water does not conduct electricity. By itself it is a poor conductor of electricity.
 Source: scienceabc.com
Source: scienceabc.com
Electricity seeks out oppositely charged particles to travel through. Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. By itself it is a poor conductor of electricity.
 Source: youtube.com
Source: youtube.com
Pure water does not conduct electricity. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. No pure water doesn t conduct electricity.
 Source: flipscience.ph
Source: flipscience.ph
When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. However water contains charged ions and impurities that make it a very good conductor of electricity. The short answer is. We re always told and taught that water conducts electricity. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater.
 Source: slideplayer.com
Source: slideplayer.com
Any impurities like salts in the water enable it to conduct electricity. By itself it is a poor conductor of electricity. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. Completely pure water is actually an insulator and cannot conduct electricity.
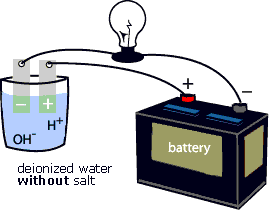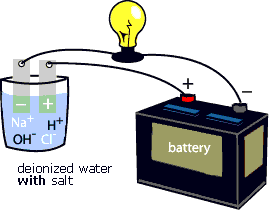 Source: w7r.blogspot.com
Source: w7r.blogspot.com
When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Completely pure water is actually an insulator and cannot conduct electricity. Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. The short answer is.
 Source: sciencealert.com
Source: sciencealert.com
Electricity seeks out oppositely charged particles to travel through. By itself it is a poor conductor of electricity. The short answer is. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Completely pure water is actually an insulator and cannot conduct electricity.
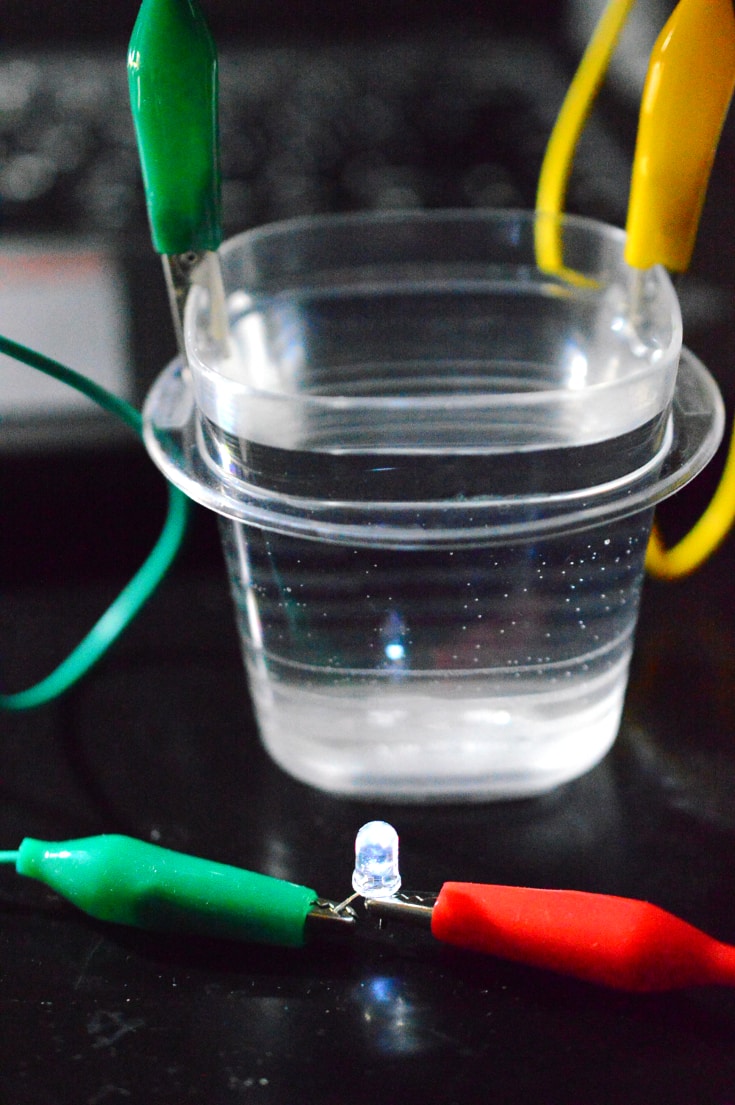 Source: rookieparenting.com
Source: rookieparenting.com
Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. No pure water doesn t conduct electricity. Electricity seeks out oppositely charged particles to travel through. Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions. Any impurities like salts in the water enable it to conduct electricity.
 Source: knowledgenuts.com
Source: knowledgenuts.com
Any impurities like salts in the water enable it to conduct electricity. Electricity seeks out oppositely charged particles to travel through. Completely pure water is actually an insulator and cannot conduct electricity. In fact this is the primary reason why water electricity is such bad news as it can cause electric shocks to those who come in contact with the dangerous pair. Pure water does not conduct electricity.
 Source: youtube.com
Source: youtube.com
Water conducts electricity because it contains dissolved matter such as minerals and chemicals with charged ions. Pure water does not conduct electricity. However water contains charged ions and impurities that make it a very good conductor of electricity. When salts are dissolved in water they separate into different electrically charged atoms called ions. Electricity seeks out oppositely charged particles to travel through.
 Source: pinclipart.com
Source: pinclipart.com
We re always told and taught that water conducts electricity. By itself it is a poor conductor of electricity. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. Completely pure water is actually an insulator and cannot conduct electricity. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title does water conduct electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






