Does sugar conduct electricity in water
Does Sugar Conduct Electricity In Water. Sugar solution does not contain free ions that are required to conduct electricity. Sugar is a molecular compound which in most cases cannot conduct electricity underwater. The water conducts so little current that it is often called a nonconductor. Does pure water conduct any electricity at.
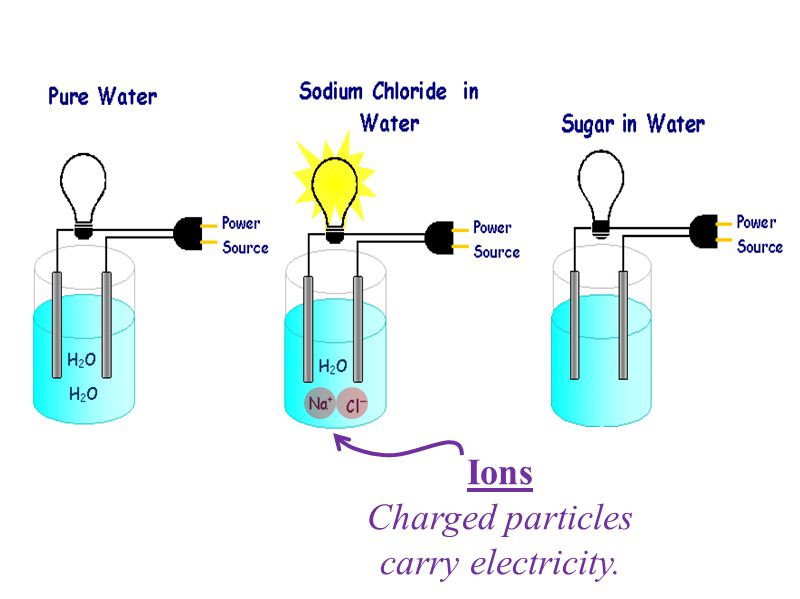 Dissolving Vs Dissociating Sugar Vs Salt Electrolytes Charged Particles Or Ions Present In A Solution Can Conduct An Electric Current Ionic Compounds Ppt Download From slideplayer.com
Dissolving Vs Dissociating Sugar Vs Salt Electrolytes Charged Particles Or Ions Present In A Solution Can Conduct An Electric Current Ionic Compounds Ppt Download From slideplayer.com
Does pure water conduct any electricity at. The salt is ionic so it can conduct electricity underwater kind of like a summary. It is the water in the sugar solution that has the ability to conduct electricity not the sugar. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions. Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. Sugar is a molecular substance and does not dissociate into positive and negative ions in a water solution therefore it does not conduct electricity.
No sugar solution does not conduct electricity.
No sugar solution does not conduct electricity. On the other hand sugar solution does not conduct an electric current because sugar c 12 h 22 o 11 dissolves in water to produce sugar molecules. Unlike electrolytic solution sugar solution does not dissociate free ions making it an insulator. So does sugar solution conduct electricity. Does sugar conduct electricity in water cause hypertension. Also i added sugar.
 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
So does sugar solution conduct electricity. The water conducts so little current that it is often called a nonconductor. Also i added sugar. The sodium chloride solution mainly consists of free sodium and chloride ions which could migrate to positively charged electrodes. In this video i test water conductivity with a simple experiment which shows how water changes its conductivity when you add salt to it.
 Source: techiescientist.com
Source: techiescientist.com
Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. The sugar cane solution is a covalent compound. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions. The salt is ionic so it can conduct electricity underwater kind of like a summary. Sugar is a molecular substance and does not dissociate into positive and negative ions in a water solution therefore it does not conduct electricity.
 Source: slideplayer.com
Source: slideplayer.com
When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. Sugar is a molecular substance and does not dissociate into positive and negative ions in a water solution therefore it does not conduct electricity. So does sugar solution conduct electricity. Unlike electrolytic solution sugar solution does not dissociate free ions making it an insulator. If tap water is used to make the sugar solution the ions in the tap water will increase the conductivity about 500 fold.
 Source: rookieparenting.com
Source: rookieparenting.com
The water conducts so little current that it is often called a nonconductor. So does sugar solution conduct electricity. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions. Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. Sugar solution does not contain free ions that are required to conduct electricity.
 Source: youtube.com
Source: youtube.com
Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. It is the water in the sugar solution that has the ability to conduct electricity not the sugar. The salt is ionic so it can conduct electricity underwater kind of like a summary. The water conducts so little current that it is often called a nonconductor.
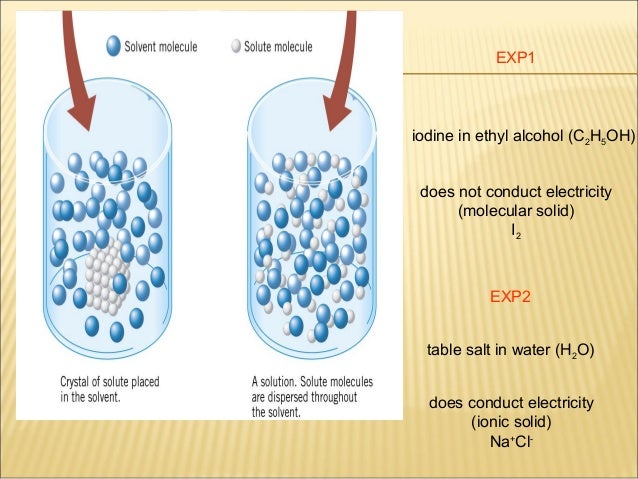 Source: slideshare.net
Source: slideshare.net
Does sugar conduct electricity in water cause hypertension. Sugar molecules are held by covalent bonds as a result they do not dissociate free ions in water. Unlike electrolytic solution sugar solution does not dissociate free ions making it an insulator. On the other hand sugar solution does not conduct an electric current because sugar c 12 h 22 o 11 dissolves in water to produce sugar molecules. Sugar is a molecular compound which in most cases cannot conduct electricity underwater.
 Source: brainly.in
Source: brainly.in
The sugar cane solution is a covalent compound. When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. Does pure water conduct any electricity at. No sugar solution does not conduct electricity. On the other hand sugar solution does not conduct an electric current because sugar c 12 h 22 o 11 dissolves in water to produce sugar molecules.
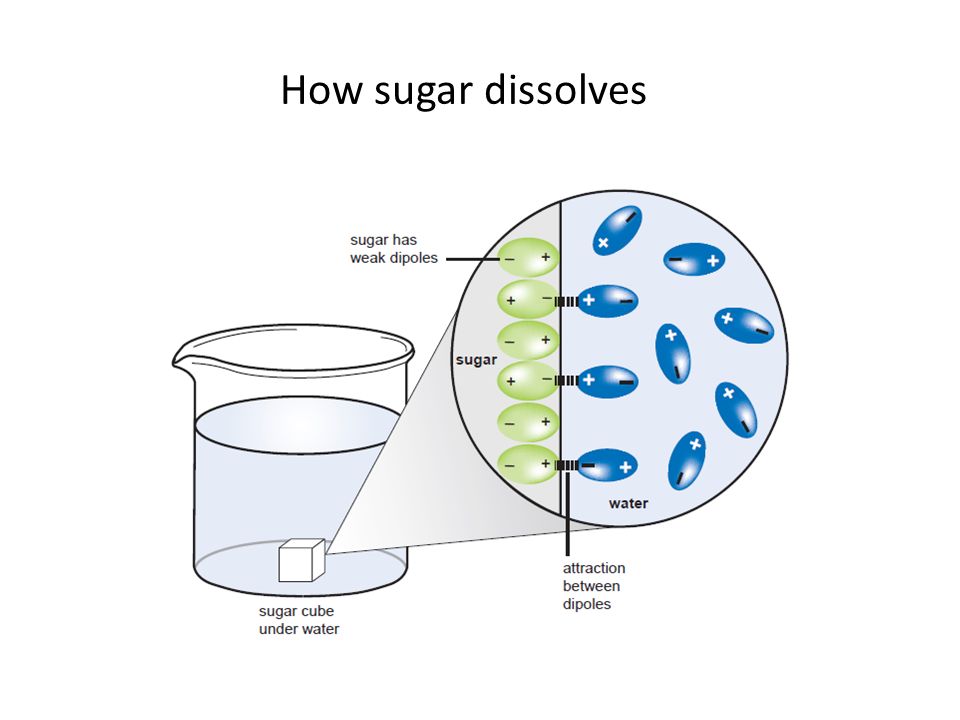 Source: slideplayer.com
Source: slideplayer.com
So does sugar solution conduct electricity. Also i added sugar. Hence sugar solution is bad conductor of electricity. It is the water in the sugar solution that has the ability to conduct electricity not the sugar. Sugar is a molecular compound which in most cases cannot conduct electricity underwater.

If tap water is used to make the sugar solution the ions in the tap water will increase the conductivity about 500 fold. Hence sugar solution is bad conductor of electricity. Sugar molecules are held by covalent bonds as a result they do not dissociate free ions in water. Does sugar conduct electricity in water cause hypertension. So does sugar solution conduct electricity.
 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
Does sugar conduct electricity in water cause hypertension. The water conducts so little current that it is often called a nonconductor. So does sugar solution conduct electricity. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions. Also i added sugar.
 Source: slideplayer.com
Source: slideplayer.com
The sugar cane solution is a covalent compound. The water conducts so little current that it is often called a nonconductor. Does pure water conduct any electricity at. Sugar is a molecular substance and does not dissociate into positive and negative ions in a water solution therefore it does not conduct electricity. Hence sugar solution is bad conductor of electricity.
 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. It is the water in the sugar solution that has the ability to conduct electricity not the sugar. Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. The sugar cane solution is a covalent compound.
 Source: youtube.com
Source: youtube.com
The sugar cane solution is a covalent compound. Sugar solution does not contain free ions that are required to conduct electricity. So does sugar solution conduct electricity. When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. If tap water is used to make the sugar solution the ions in the tap water will increase the conductivity about 500 fold.

Sugar solution does not contain free ions that are required to conduct electricity. The water conducts so little current that it is often called a nonconductor. Sugar solution does not contain free ions that are required to conduct electricity. It is the water in the sugar solution that has the ability to conduct electricity not the sugar. In this video i test water conductivity with a simple experiment which shows how water changes its conductivity when you add salt to it.
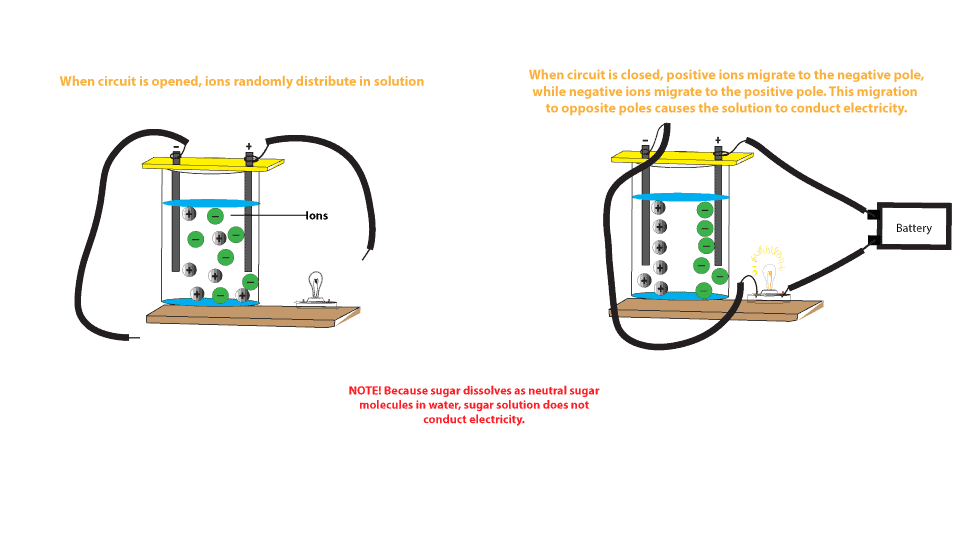 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
When it is dissolved in water does not dissociate to give free ions which could migrate to cathode or anode. Sugar is a molecular compound which in most cases cannot conduct electricity underwater. These sugar molecules are usually neutral not charged and so are unable to move to the opposite ends of the electrodes like the ions. Dka can also be caused by an underlying illness such as an infection or a severe stomach bug that causes vomiting. Also i added sugar.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title does sugar conduct electricity in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





