Does salt water conduct more electricity than freshwater
Does Salt Water Conduct More Electricity Than Freshwater. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. When salts are dissolved in water they separate into different electrically charged atoms called ions. Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors.
 This New Technology Generates Electricity When Fresh Water Meets Saltwater From interestingengineering.com
This New Technology Generates Electricity When Fresh Water Meets Saltwater From interestingengineering.com
Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity which type of water conducts electricity salt water or fresh. Salt molecules are made of sodium ions and chlorine ions. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. Concentrations are not to be exceeded more than 10 of the time during any one year period. As the ions float freely they carry electricity through the water making the water conducive.
This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy.
Concentrations are not to be exceeded more than 10 of the time during any one year period. To test the conductivity of salt water vs fresh water we can perform a simple and fun experiment. Electricity is a steady flow of electrons or electrically charged particles through a substance. The flow of rivers into estuaries. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity which type of water conducts electricity salt water or fresh. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity.
 Source: phys.org
Source: phys.org
This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Concentrations are not to be exceeded more than 10 of the time during any one year period. To test the conductivity of salt water vs fresh water we can perform a simple and fun experiment. Most of the water in the world contains high enough levels of salt to prevent it from being classified as fresh water. True fresh water bodies are much rarer than salt water bodies.
 Source: theconversation.com
Source: theconversation.com
To understand why salt water conducts electricity we have to first understand what electricity is. To test the conductivity of salt water vs fresh water we can perform a simple and fun experiment. Any impurities like salts in the water enable it to conduct electricity. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
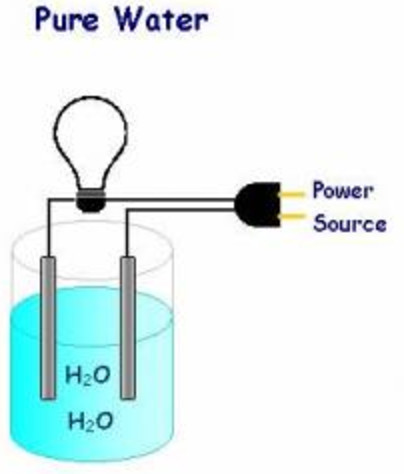 Source: classnotes.org.in
Source: classnotes.org.in
Salt water is literally dangerous to drink. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. True fresh water bodies are much rarer than salt water bodies. Because of salt water intrusion during high tides. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity.
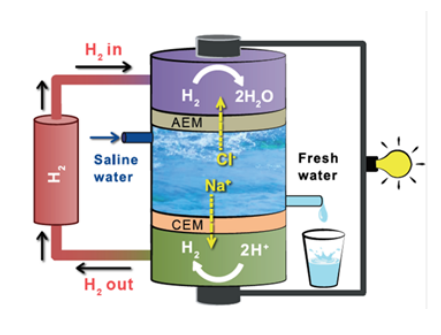 Source: iiserpunenews.wordpress.com
Source: iiserpunenews.wordpress.com
Pure water does not conduct electricity. As the ions float freely they carry electricity through the water making the water conducive. To understand why salt water conducts electricity we have to first understand what electricity is. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
 Source: interestingengineering.com
Source: interestingengineering.com
When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. When salts are dissolved in water they separate into different electrically charged atoms called ions. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. Concentrations are not to be exceeded more than 10 of the time during any one year period. For both freshwater and mineralized water the higher the flow volume the more it will affect salinity and conductivity 29.
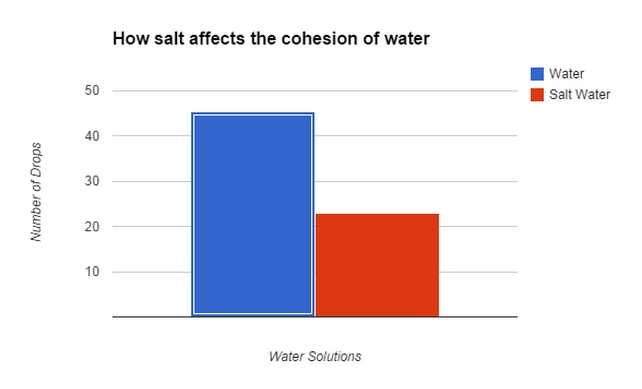 Source: vhmsscience.weebly.com
Source: vhmsscience.weebly.com
Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Salt water is literally dangerous to drink. As the ions float freely they carry electricity through the water making the water conducive. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater.
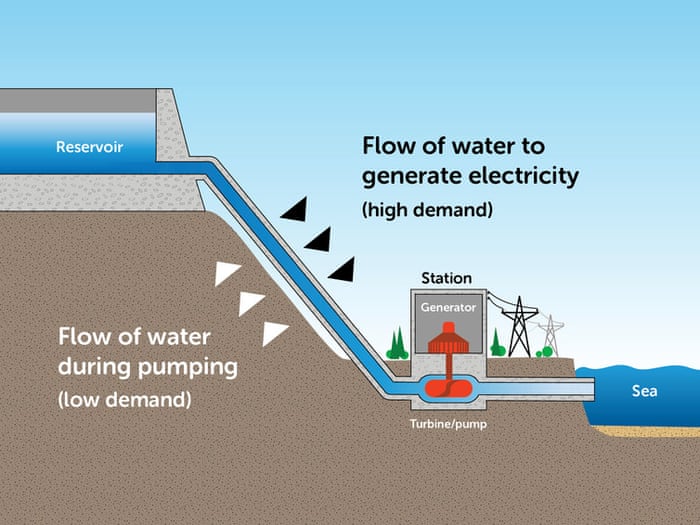 Source: theguardian.com
Source: theguardian.com
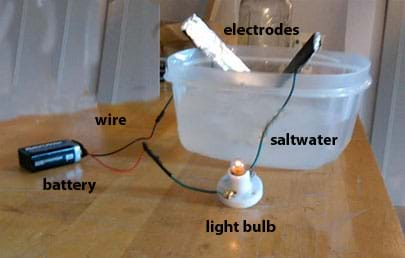
What you ll need to experiment with the conductivity of salt water. Electricity is a steady flow of electrons or electrically charged particles through a substance. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity. This means that electricity flows through salt water more readily and efficiently than it does through fresh water. Most of the water in the world contains high enough levels of salt to prevent it from being classified as fresh water.
 Source: aslo.org
Source: aslo.org
Concentrations are not to be exceeded more than 10 of the time during any one year period. Concentrations are not to be exceeded more than 10 of the time during any one year period. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity. Because of salt water intrusion during high tides. Most of the water in the world contains high enough levels of salt to prevent it from being classified as fresh water.
 Source: teachengineering.org
Source: teachengineering.org
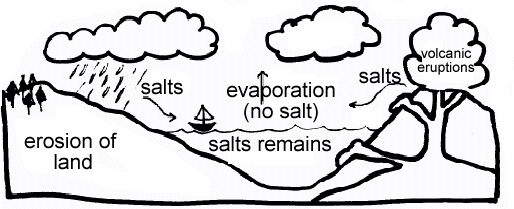
Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. The short answer is. Salt water is literally dangerous to drink. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. Electricity is a steady flow of electrons or electrically charged particles through a substance.
 Source: msnucleus.org
Source: msnucleus.org
For both freshwater and mineralized water the higher the flow volume the more it will affect salinity and conductivity 29. Salt molecules are made of sodium ions and chlorine ions. As the ions float freely they carry electricity through the water making the water conducive. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. To understand why salt water conducts electricity we have to first understand what electricity is.
 Source: rookieparenting.com
Source: rookieparenting.com
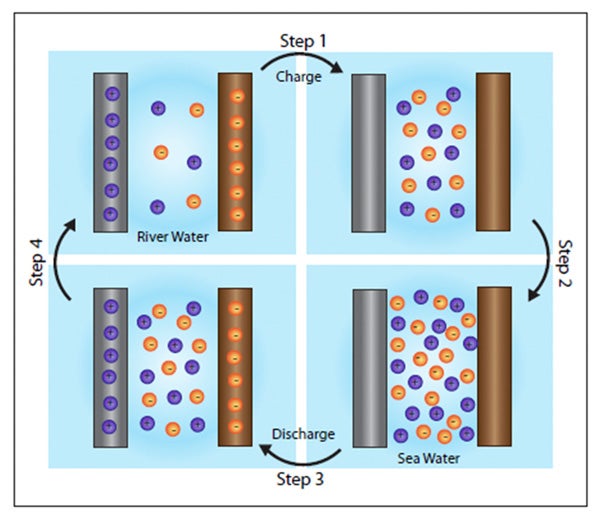
When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. To understand why salt water conducts electricity we have to first understand what electricity is. Salt water is literally dangerous to drink. To test the conductivity of salt water vs fresh water we can perform a simple and fun experiment. Rain itself can have a higher conductivity than pure water due to the incorporation of gases and dust particles 23.
 Source: news.stanford.edu
Source: news.stanford.edu
Salt molecules are made of sodium ions and chlorine ions. Salt water conducts more electricity than fresh water because it contains great amounts of ions which can conduct a lot of electricity. Concentrations are not to be exceeded more than 10 of the time during any one year period. True fresh water bodies are much rarer than salt water bodies. Salt molecules are made of sodium ions and chlorine ions.
 Source: interestingengineering.com
Source: interestingengineering.com
To understand why salt water conducts electricity we have to first understand what electricity is. Rain itself can have a higher conductivity than pure water due to the incorporation of gases and dust particles 23. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. The flow of rivers into estuaries. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water.
 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
As the ions float freely they carry electricity through the water making the water conducive. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Electricity is a steady flow of electrons or electrically charged particles through a substance. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Salt molecules are made of sodium ions and chlorine ions.
 Source: rookieparenting.com
Source: rookieparenting.com
When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water. Conductivity is the ability of water to conduct an electrical current and the dissolved ions are the conductors. Even a small amount of ions in a water solution makes it able to conduct electricity so definitely don t add salt to your lightning storm bathwater. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. When water contains these ions it will conduct electricity such as from a lightning bolt or a wire from the wall socket as the electricity from the source will seek out oppositely charged ions in the water.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title does salt water conduct more electricity than freshwater by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





