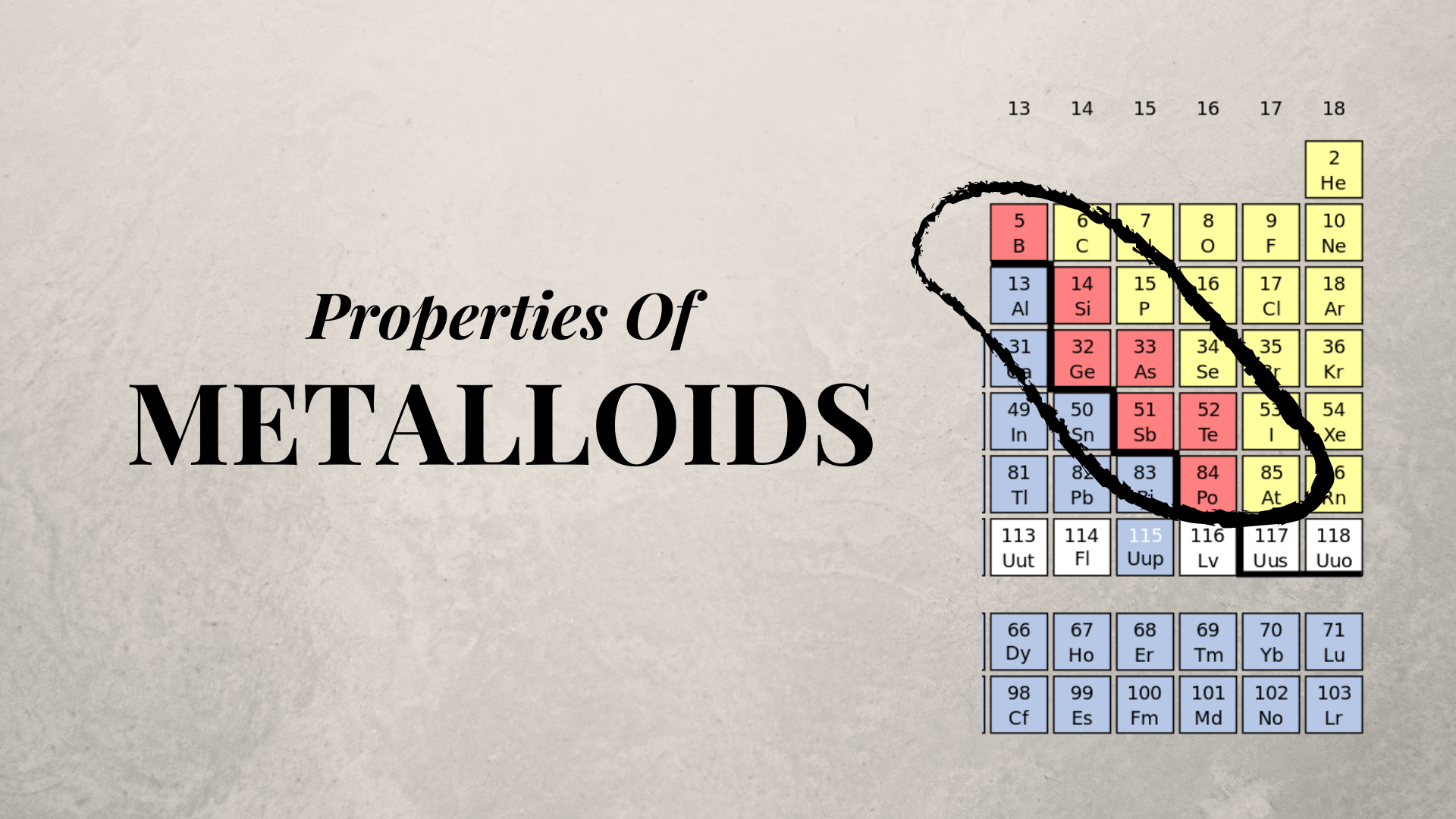
Describe metalloids characteristic properties
Describe Metalloids Characteristic Properties. The metalloids are boron silicon germanium arsenic antimony and tellurium. In general metalloids have a metallic luster. Metalloids have semiconductor properties and form amphoteric oxides. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table.
 4 Properties Of Metalloids Science Trends From sciencetrends.com
4 Properties Of Metalloids Science Trends From sciencetrends.com
Physical properties include things like the freezing point and density. Despite the lack of specificity the term remains in use in the literature of chemistry. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. Physical properties of metalloids are as follows.
Metalloids have semiconductor properties and form amphoteric oxides.
In general metalloids have a metallic luster. Physical properties include things like the freezing point and density. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Despite the lack of specificity the term remains in use in the literature of chemistry. In general metalloids have a metallic luster.
 Source: slideplayer.com
Source: slideplayer.com
Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. The six commonly recognised metalloids are boron. Physical properties of metalloids are as follows.
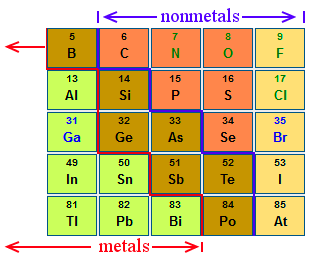 Source: chemicool.com
Source: chemicool.com
A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. Despite the lack of specificity the term remains in use in the literature of chemistry. Physical properties include things like the freezing point and density.
 Source: numerade.com
Source: numerade.com
Despite the lack of specificity the term remains in use in the literature of chemistry. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. The six commonly recognised metalloids are boron. Despite the lack of specificity the term remains in use in the literature of chemistry. Physical properties of metalloids are as follows.
 Source: thoughtco.com
Source: thoughtco.com
The metalloids are boron silicon germanium arsenic antimony and tellurium. Metalloids have low elasticity they are very brittle. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. The metalloids are boron silicon germanium arsenic antimony and tellurium.
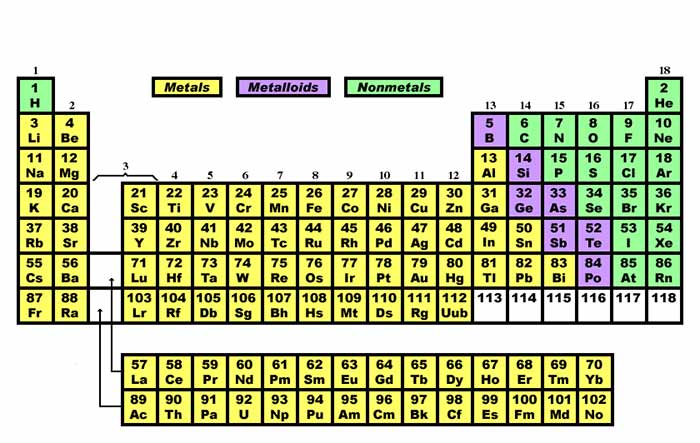 Source: texasgateway.org
Source: texasgateway.org
Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. In general metalloids have a metallic luster. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table.
 Source: sciencetrends.com
Source: sciencetrends.com
Metalloids have semiconductor properties and form amphoteric oxides. In general metalloids have a metallic luster. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Physical properties of metalloids are as follows.
 Source: pinterest.com
Source: pinterest.com
A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Metalloids have low elasticity they are very brittle. The metalloids are boron silicon germanium arsenic antimony and tellurium. In general metalloids have a metallic luster. Metalloids have a solid state of matter.
 Source: quora.com
Source: quora.com
Physical properties include things like the freezing point and density. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. In general metalloids have a metallic luster. Physical properties of metalloids are as follows. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
Physical properties of metalloids are as follows. In general metalloids have a metallic luster. Physical properties of metalloids are as follows. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties.
 Source: keslerscience.com
Source: keslerscience.com
Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Physical properties of metalloids are as follows. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties and form amphoteric oxides.
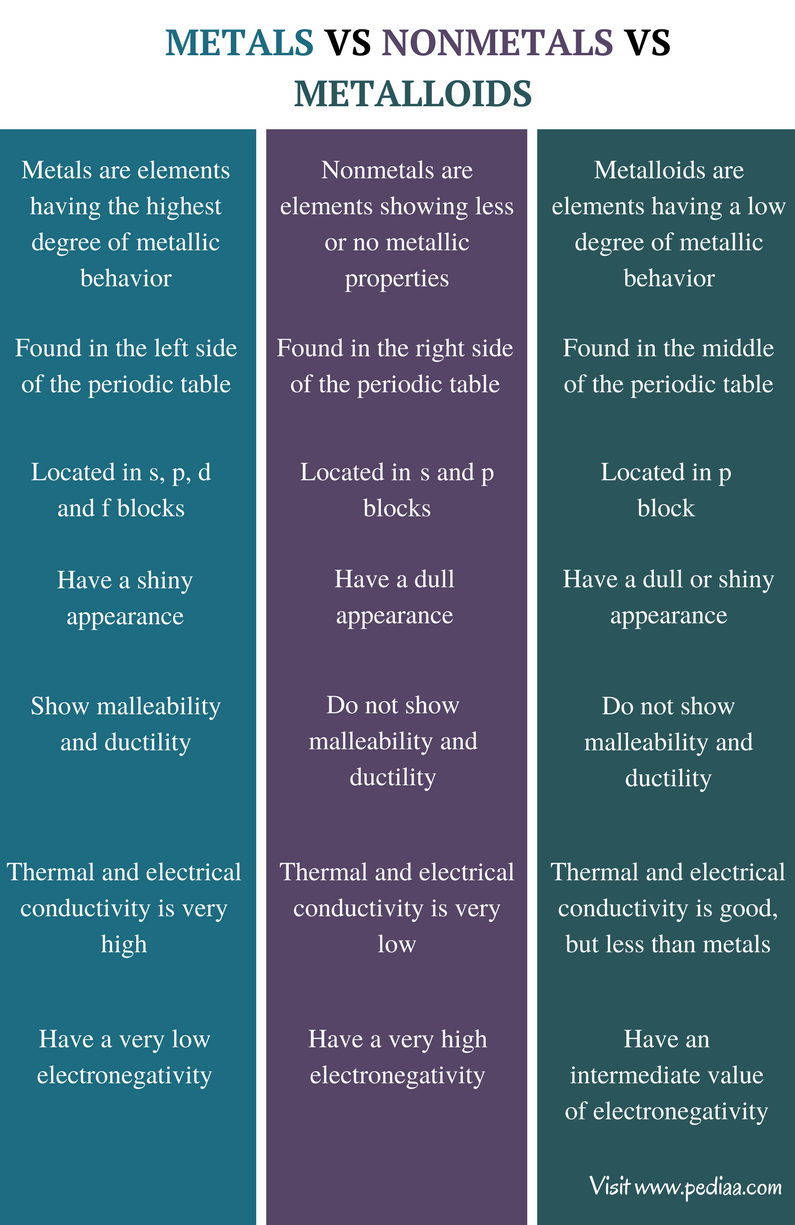 Source: pediaa.com
Source: pediaa.com
In general metalloids have a metallic luster. Physical properties include things like the freezing point and density. Despite the lack of specificity the term remains in use in the literature of chemistry. Physical properties of metalloids are as follows. Metalloids have low elasticity they are very brittle.
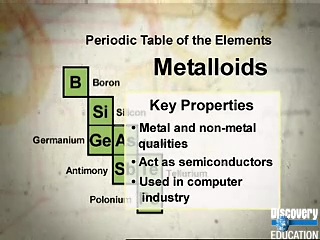 Source: texasgateway.org
Source: texasgateway.org
Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Despite the lack of specificity the term remains in use in the literature of chemistry. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. The six commonly recognised metalloids are boron.
 Source: study.com
Source: study.com
Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. Metalloids have semiconductor properties and form amphoteric oxides. Physical properties of metalloids are as follows. The metalloids are boron silicon germanium arsenic antimony and tellurium. Metalloids have low elasticity they are very brittle.

Despite the lack of specificity the term remains in use in the literature of chemistry. Most metalloids have a shiny metallic appearance but are brittle unexceptional electrical conductors and display nonmetallic chemical properties. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Physical properties are characteristics that can be documented or observed without altering the substance of the element without changing the group of molecules into substances. In general metalloids have a metallic luster.
 Source: slideplayer.com
Source: slideplayer.com
Metalloids have low elasticity they are very brittle. Physical properties of metalloids are as follows. Describe the preparation properties and compounds of boron and silicon a series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Physical properties include things like the freezing point and density. A metalloid is a type of chemical element which has a preponderance of properties in between or that are a mixture of those of metals and nonmetals there is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title describe metalloids characteristic properties by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.