Describe how hydrogen bonding accounts for the properties of water
Describe How Hydrogen Bonding Accounts For The Properties Of Water. Salt na cl dissolves in water. Cohesion is the ability of water to stick to itself. ø the hydrogen bonds in water molecule are responsible for the unusual properties of water. Cohesion adhesion and surface tension.
 Pdf Water Structure And Properties From researchgate.net
Pdf Water Structure And Properties From researchgate.net
ø intermolecular hydrogen bonds are responsible for secondary and tertiary structure of proteins and nucleic acids. Cohesion adhesion and surface tension. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. Furthermore water molecules interact through hydrogen bonding to create 3 very unique properties. The hydrogen bonding between water molecules means they are more likely to stick together than break apart. Water is a polar molecule that has a high level of polarity and attraction to ions and other polar molecules.
In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them.
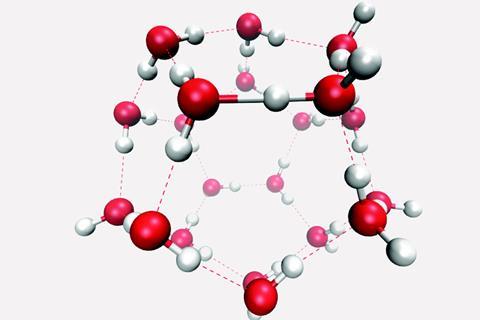
Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar. Therefore hydrogen bonding arises in water molecules due to the dipole dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another h 2 o molecule. Here the location of the bond pair of electrons in the o h bond is very close to the oxygen nucleus due to the large difference in the electronegativities of oxygen and hydrogen. ø the hydrogen bonds in water molecule are responsible for the unusual properties of water. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. Hydrogen bonding in liquid water hydrogen bonds are relatively weak but since there are so many of them present in water they determine its chemical properties to a large degree.
 Source: studocu.com
Source: studocu.com
Water is a polar molecule that has a high level of polarity and attraction to ions and other polar molecules. All of the electron pairs shared and unshared repel each other. These bonds are primarily the electrical attractions between positively charged hydrogen atoms and negatively charged oxygen atoms. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them.
 Source: biology-pages.info
Source: biology-pages.info
ø intermolecular hydrogen bonds are responsible for secondary and tertiary structure of proteins and nucleic acids. Therefore hydrogen bonding arises in water molecules due to the dipole dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another h 2 o molecule. Hydrogen bonding in liquid water hydrogen bonds are relatively weak but since there are so many of them present in water they determine its chemical properties to a large degree. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. In h 2 o only two of the six outer shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non bonding pairs.
 Source: chegg.com
Source: chegg.com
Cohesion adhesion and surface tension. The hydrogen bonding between water molecules means they are more likely to stick together than break apart. ø intermolecular hydrogen bonds are responsible for the high boiling point of water 100oc. Here the location of the bond pair of electrons in the o h bond is very close to the oxygen nucleus due to the large difference in the electronegativities of oxygen and hydrogen. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent.
 Source: khanacademy.org
Source: khanacademy.org
Cohesion is the ability of water to stick to itself. This is called cohesion. In h 2 o only two of the six outer shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non bonding pairs. When water form hydrogen bonds with other substance the attraction is called adhesion. Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar.
 Source: studylib.net
Source: studylib.net
Study offline without internet. In h 2 o only two of the six outer shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non bonding pairs. Study offline without internet. The hydrogen bonding between water molecules means they are more likely to stick together than break apart. Therefore hydrogen bonding arises in water molecules due to the dipole dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another h 2 o molecule.
 Source: chemistryworld.com
Source: chemistryworld.com
Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar. In h 2 o only two of the six outer shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non bonding pairs. ø the hydrogen bonds in water molecule are responsible for the unusual properties of water. When water form hydrogen bonds with other substance the attraction is called adhesion. Salt na cl dissolves in water.
 Source: ib.bioninja.com.au
Source: ib.bioninja.com.au
Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar. Hydrogen bonding in liquid water hydrogen bonds are relatively weak but since there are so many of them present in water they determine its chemical properties to a large degree. Water can form hydrogen bonds which make it a powerful solvent. Here the location of the bond pair of electrons in the o h bond is very close to the oxygen nucleus due to the large difference in the electronegativities of oxygen and hydrogen. Study offline without internet.
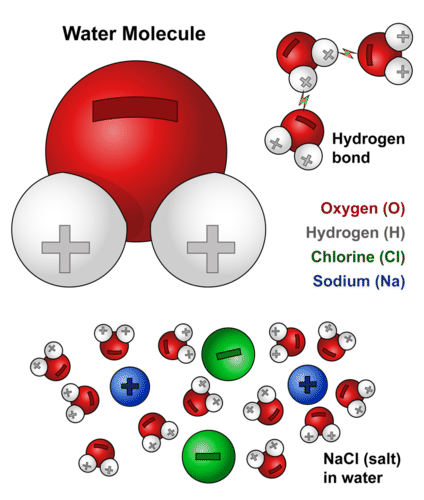
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. Because of hydrogen bonds water molecules develop strong intermolecular attraction between them. Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar. Study offline without internet.
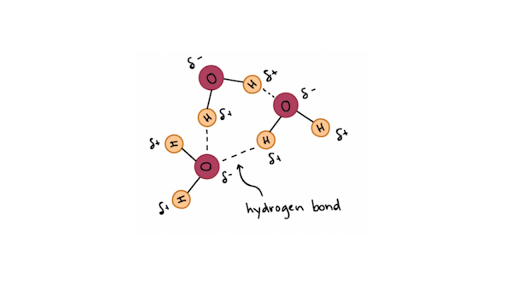
ø intermolecular hydrogen bonds are responsible for the high boiling point of water 100oc. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. All of the electron pairs shared and unshared repel each other. Hydrogen bonding in liquid water hydrogen bonds are relatively weak but since there are so many of them present in water they determine its chemical properties to a large degree.
 Source: researchgate.net
Source: researchgate.net
Because of hydrogen bonds water molecules develop strong intermolecular attraction between them. Water is highly cohesive and adhesive. Cohesion adhesion and surface tension. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. Therefore hydrogen bonding arises in water molecules due to the dipole dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another h 2 o molecule.
 Source: chegg.com
Source: chegg.com
Because of hydrogen bonds water molecules develop strong intermolecular attraction between them. Water is a polar molecule that has a high level of polarity and attraction to ions and other polar molecules. When water form hydrogen bonds with other substance the attraction is called adhesion. Water is highly cohesive and adhesive. Therefore hydrogen bonding arises in water molecules due to the dipole dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another h 2 o molecule.
 Source: slideshare.net
Source: slideshare.net
Water can form hydrogen bonds which make it a powerful solvent. Study offline without internet. ø intermolecular hydrogen bonds are responsible for secondary and tertiary structure of proteins and nucleic acids. Because of hydrogen bonds water molecules develop strong intermolecular attraction between them. Here the location of the bond pair of electrons in the o h bond is very close to the oxygen nucleus due to the large difference in the electronegativities of oxygen and hydrogen.
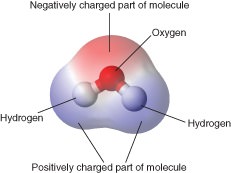
Salt na cl dissolves in water. This is called cohesion. Furthermore water molecules interact through hydrogen bonding to create 3 very unique properties. Water molecules are attracted to other molecules that contain a full charge like an ion a partial charge or polar. Cohesion adhesion and surface tension.
 Source: chegg.com
Source: chegg.com
ø intermolecular hydrogen bonds are responsible for the high boiling point of water 100oc. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. ø intermolecular hydrogen bonds are responsible for secondary and tertiary structure of proteins and nucleic acids. Cohesion is the ability of water to stick to itself. Here the location of the bond pair of electrons in the o h bond is very close to the oxygen nucleus due to the large difference in the electronegativities of oxygen and hydrogen.
Source: quora.com
ø intermolecular hydrogen bonds are responsible for secondary and tertiary structure of proteins and nucleic acids. These bonds are primarily the electrical attractions between positively charged hydrogen atoms and negatively charged oxygen atoms. All of the electron pairs shared and unshared repel each other. Water is a polar molecule that has a high level of polarity and attraction to ions and other polar molecules. Water can form hydrogen bonds which make it a powerful solvent.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title describe how hydrogen bonding accounts for the properties of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.