Copper sulfate electroplating
Copper Sulfate Electroplating. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. Copper sulfate is the main source of copper ions in an electroplating solution. When the current is flowing oxidation loss of electrons happens at the copper anode adding copper ions to the solution. The electrolyte bath contains three primary inorganic components.
 Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
Electrolysis Copper Sulfate Solution With Copper Carbon Graphite Electrodes Electroplating Half Equations Products Anode Cathode Apparatus Electrolyte Cell Gcse Chemistry Ks4 Science Igcse O Level Revision Notes From docbrown.info
So it can be concluded that during electrolysis of copper sulfate with copper electrodes. First let s know something about electroplating. Therefore the blue colour of the cu 2 ions stays constant because cu deposited cu dissolved. In this practical students carry out the electrolysis of copper ii sulfate solution. You do not want to have an overly saturated copper acetate solution. When copper ii sulfate is electrolysed with a copper anode electrode the cathode can be carbon or copper the copper deposit on the cathode equals the copper dissolves at the anode.
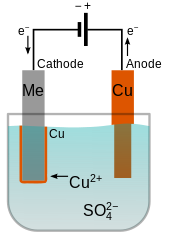
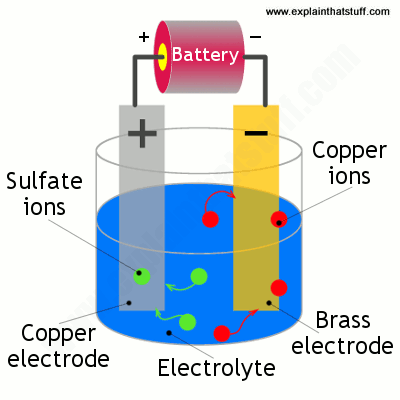
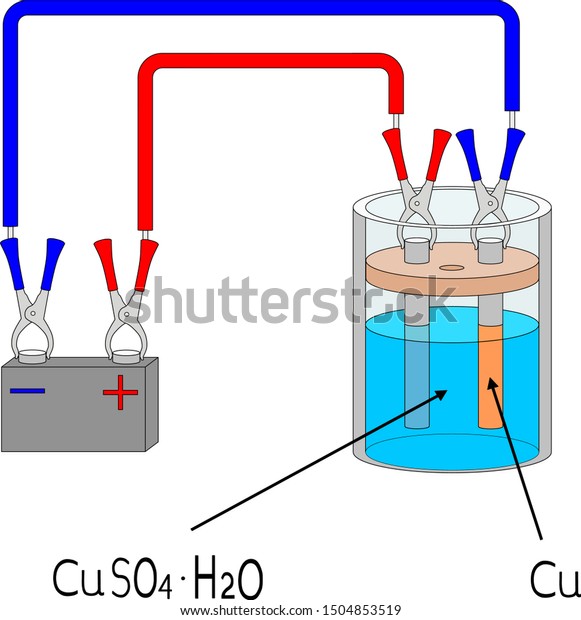
The copper sulfate solution is an electrolyte that conducts electricity from one electrode to the other creating an electrical current.
Copper sulfate is the main source of copper ions in an electroplating solution. When the concentration of copper sulfate is too low the deposition rate of copper is slow. In this process an electric current is used to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. You do not want to have an overly saturated copper acetate solution. The copper sulfate solution is an electrolyte that conducts electricity from one electrode to the other creating an electrical current.
 Source: rfcafe.com
Source: rfcafe.com
Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Therefore the blue colour of the cu 2 ions stays constant because cu deposited cu dissolved. You do not want to have an overly saturated copper acetate solution. Hello friends we are going to do simple copper electroplating using copper sulfate. When we start electroplating we want the copper atoms to create very thin even layers on our object.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In this process an electric current is used to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. A high copper and low acid system is usually used in electroplating hole filling. At the same time so 4 reacts with copper anode and becomes cuso 4 but in water it can not exist as single molecules instead of that cuso 4 will split into cu so 4 and dissolve in water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating.
 Source: docbrown.info
Source: docbrown.info
The object to be plated is placed at the cathode. A high copper and low acid system is usually used in electroplating hole filling. When we start electroplating we want the copper atoms to create very thin even layers on our object. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. When the current is flowing oxidation loss of electrons happens at the copper anode adding copper ions to the solution.
 Source: explainthatstuff.com
Source: explainthatstuff.com
In this process an electric current is used to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. A quick tip her. When we start electroplating we want the copper atoms to create very thin even layers on our object. The copper sulfate solution is an electrolyte that conducts electricity from one electrode to the other creating an electrical current. The copper coating is an important component of.
 Source: aven.amritalearning.com
Source: aven.amritalearning.com
Therefore the blue colour of the cu 2 ions stays constant because cu deposited cu dissolved. The copper coating is an important component of. When the current is flowing oxidation loss of electrons happens at the copper anode adding copper ions to the solution. Copper sulfate is the main source of copper ions in an electroplating solution. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper.
 Source: m.youtube.com
Source: m.youtube.com
First let s know something about electroplating. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Copper sulfate is the main source of copper ions in an electroplating solution. This class experiment can be done by students working either in pairs or threes.
 Source: shutterstock.com
Source: shutterstock.com
In this process an electric current is used to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode. In fact it is far better to have a weaker solution than a stronger one. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. In the above process after taking electrons the neutral copper atoms get deposited on the cathode. Procedure two 250 ml beakers stir bar magnetic stirrer vernier constant current system 1 cm x 10 cm strip of copper for the anode.
 Source: yenka.com
Source: yenka.com
When we start electroplating we want the copper atoms to create very thin even layers on our object. The electrolyte is 1 0 m copper sulfate in 1 0 m sulfuric acid. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. In fact it is far better to have a weaker solution than a stronger one. The copper coating is an important component of.

Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. So it can be concluded that during electrolysis of copper sulfate with copper electrodes. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. First let s know something about electroplating.
 Source: m.youtube.com
Source: m.youtube.com
At the same time so 4 reacts with copper anode and becomes cuso 4 but in water it can not exist as single molecules instead of that cuso 4 will split into cu so 4 and dissolve in water. The electrolyte is 1 0 m copper sulfate in 1 0 m sulfuric acid. Hello friends we are going to do simple copper electroplating using copper sulfate. When copper ii sulfate is electrolysed with a copper anode electrode the cathode can be carbon or copper the copper deposit on the cathode equals the copper dissolves at the anode. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper.
 Source: docbrown.info
Source: docbrown.info
Procedure two 250 ml beakers stir bar magnetic stirrer vernier constant current system 1 cm x 10 cm strip of copper for the anode. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. This class experiment can be done by students working either in pairs or threes. The anode is a strip of copper. When the current is flowing oxidation loss of electrons happens at the copper anode adding copper ions to the solution.
 Source: docbrown.info
Source: docbrown.info
The electrolyte bath contains three primary inorganic components. In fact it is far better to have a weaker solution than a stronger one. When the concentration of copper sulfate is too low the deposition rate of copper is slow. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. In this practical students carry out the electrolysis of copper ii sulfate solution.
 Source: docbrown.info
Source: docbrown.info
In fact it is far better to have a weaker solution than a stronger one. At the same time so 4 reacts with copper anode and becomes cuso 4 but in water it can not exist as single molecules instead of that cuso 4 will split into cu so 4 and dissolve in water. When the current is flowing oxidation loss of electrons happens at the copper anode adding copper ions to the solution. In the above process after taking electrons the neutral copper atoms get deposited on the cathode. Procedure two 250 ml beakers stir bar magnetic stirrer vernier constant current system 1 cm x 10 cm strip of copper for the anode.
 Source: chemedx.org
Source: chemedx.org
So it can be concluded that during electrolysis of copper sulfate with copper electrodes. Therefore the blue colour of the cu 2 ions stays constant because cu deposited cu dissolved. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. The electrolyte bath contains three primary inorganic components. A quick tip her.
 Source: quora.com
Source: quora.com
In fact it is far better to have a weaker solution than a stronger one. In the above process after taking electrons the neutral copper atoms get deposited on the cathode. Electroplating is the process of plating a metal onto the other by hydrolysis mostly to prevent corrosion of metal or decorative purposes. Copper sulfate is the main source of copper ions in an electroplating solution. You do not want to have an overly saturated copper acetate solution.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title copper sulfate electroplating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.