Copper sulfate electrolysis
Copper Sulfate Electrolysis. Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. Electrolysis of copper sulfate. Electricity is passed through solutions containing copper compounds such as copper ii sulfate. In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode.
 Principle Of Electrolysis Of Copper Sulfate Electrolyte Electrical4u From electrical4u.com
Principle Of Electrolysis Of Copper Sulfate Electrolyte Electrical4u From electrical4u.com
Electrolysis of copper ii sulphate solution this experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. Copper is purified by electrolysis. Electrolysis of copper sulfate. The electrical energy comes from a d c. In this practical students carry out the electrolysis of copper ii sulfate solution. That means that how much the anode has lost the cathode should have gained.
The anode is positively charged ions and the cathode are negatively charged ions.
Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. Copper is purified by electrolysis. Direct current battery or power pack supply. Copper electrodes take part in the reactions and are described as non inert. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The anode is positively charged ions and the cathode are negatively charged ions.
 Source: electrical4u.com
Source: electrical4u.com
Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. This class experiment can be done by students working either in pairs or threes. In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. Electrolysis of copper sulfate.

Copper sulfate is toxic and sulfuric acid is corrosive wear gloves when handling them. Electrolysis of copper sulfate. Electrolysis of copper ii sulphate solution this experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. To investigate the electrolysis of copper sulfate solution using non inert electrodes.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes. The use of copper electrodes illustrates how copper is refined industrially. Electrolysis of copper ii sulphate solution this experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. Electrolysis of copper sulfate is a way of splitting up decomposition of the compound copper sulfate using electrical energy.
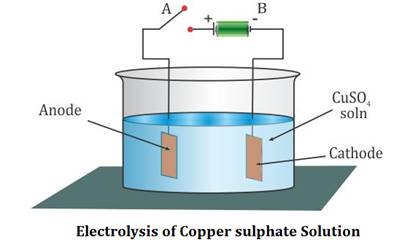 Source: topperlearning.com
Source: topperlearning.com
The use of copper electrodes illustrates how copper is refined industrially. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The anode positive electrode is made from impure copper and. That means that how much the anode has lost the cathode should have gained.
 Source: docbrown.info
Source: docbrown.info
Direct current battery or power pack supply. Copper is purified by electrolysis. Electricity is passed through solutions containing copper compounds such as copper ii sulfate. The electrical energy comes from a d c. Direct current battery or power pack supply.
 Source: docbrown.info
Source: docbrown.info
The procedure is extremely simple just get a copper sulfate solution insert two electrodes and run a current through them. Electrolysis of copper ii sulphate solution this experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. Copper sulfate is toxic and sulfuric acid is corrosive wear gloves when handling them. Electrolysis of copper sulfate is a way of splitting up decomposition of the compound copper sulfate using electrical energy. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper.

Copper electrodes take part in the reactions and are described as non inert. The electrical energy comes from a d c. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. This class experiment can be done by students working either in pairs or threes. In this practical students carry out the electrolysis of copper ii sulfate solution.
 Source: connect.collins.co.uk
Source: connect.collins.co.uk
At the positive electrode copper. Direct current battery or power pack supply. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. Copper electrodes take part in the reactions and are described as non inert. That means that how much the anode has lost the cathode should have gained.
 Source: m.youtube.com
Source: m.youtube.com
The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. Electricity is passed through solutions containing copper compounds such as copper ii sulfate. Direct current battery or power pack supply. Electrolysis of copper sulfate. In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode.
 Source: docbrown.info
Source: docbrown.info
In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. As cuso 4 is an electrolyte it splits into cu cation and so 4 anion ions and move freely in the solution. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. To investigate the electrolysis of copper sulfate solution using non inert electrodes. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper.
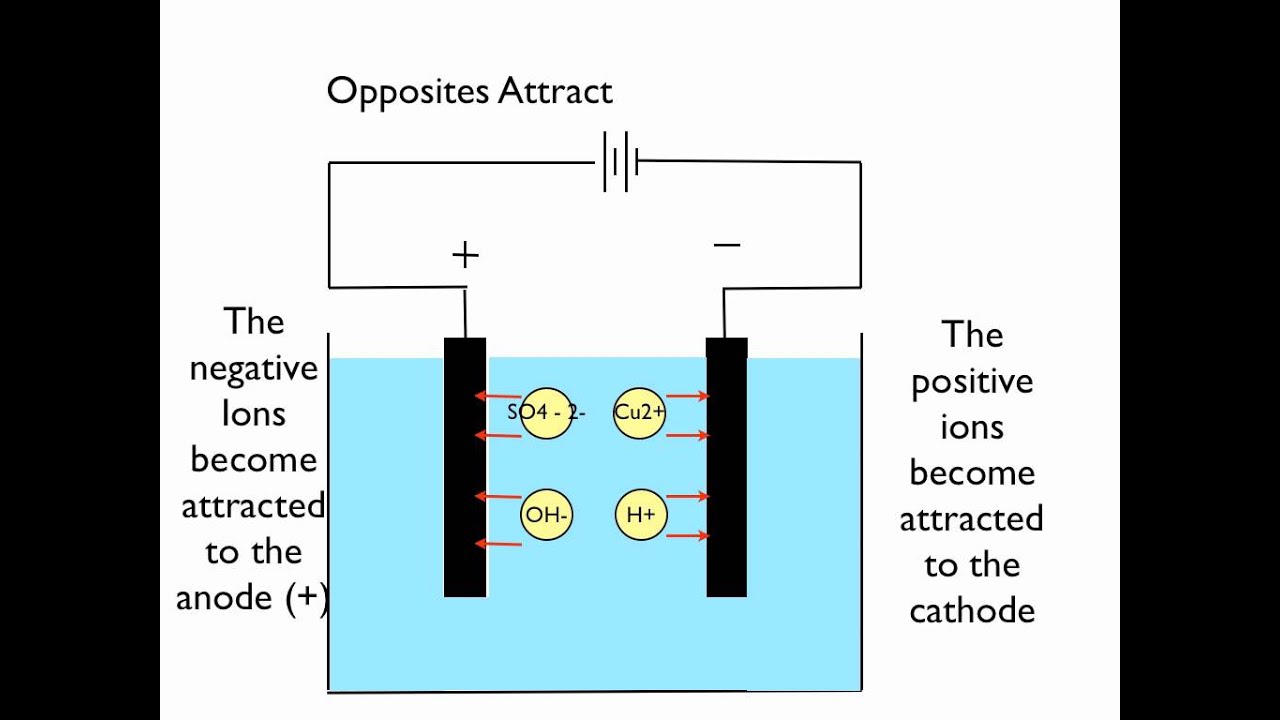 Source: m.youtube.com
Source: m.youtube.com
To investigate the electrolysis of copper sulfate solution using non inert electrodes. The use of copper electrodes illustrates how copper is refined industrially. Direct current battery or power pack supply. Copper is purified by electrolysis. The electrical energy comes from a d c.
 Source: researchgate.net
Source: researchgate.net
Whenever copper sulfate or cuso 4 is added to water it gets dissolved in the water. At the positive electrode copper. This class experiment can be done by students working either in pairs or threes. Electrolysis of copper sulfate. Electrolysis of copper ii sulphate solution this experiment is designed to demonstrate the different products obtained when the electrolysis of copper ii sulfate solution is carried out first with inert graphite electrodes and then with copper electrodes.

Electricity is passed through solutions containing copper compounds such as copper ii sulfate. In the electrolysis of cupper ii electrodes and copper sulphate solution the copper ions at the anode give ions to the cathode. The electrical energy comes from a d c. The anode positive electrode is made from impure copper and. The use of copper electrodes illustrates how copper is refined industrially.
 Source: pinterest.com
Source: pinterest.com
The use of copper electrodes illustrates how copper is refined industrially. The electrical energy comes from a d c. Electrolysis of copper ii sulphate solution this experiment enables students to carry out the electrolysis of copper ii sulfate solution and to link their findings with the industrial electrolytic refining of copper. The anode is positively charged ions and the cathode are negatively charged ions. Copper is purified by electrolysis.
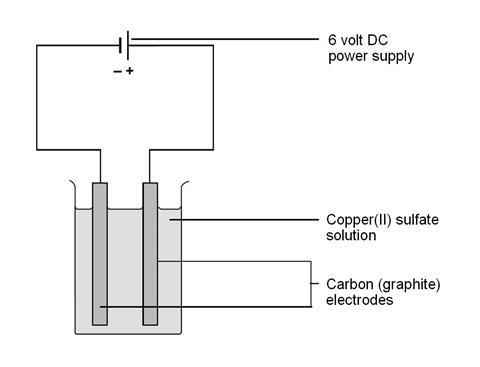 Source: edu.rsc.org
Source: edu.rsc.org
Direct current battery or power pack supply. To investigate the electrolysis of copper sulfate solution using non inert electrodes. The anode the positive electrode must be an inert material that can withstand extremely oxidizing conditions. The outcomes of the experiment provide the opportunity to introduce a discussion about electroplating and the industrial electrolytic refining of copper. The electrical energy comes from a d c.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title copper sulfate electrolysis by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.