Copper plating iron
Copper Plating Iron. Placement of a metal such as an iron nail in the lemon bath containing suspended copper ions results in a simple copper plating of the iron nail. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. The primary reason for copper plating is to improve the conductivity of a substrate copper is highly conductive only silver is a more effective metal at conducting electricity. The uses of copper plating.
 What Is The Chemical Equation For Copper Electroplating Using The Solution Of Cuso4 Quora From quora.com
What Is The Chemical Equation For Copper Electroplating Using The Solution Of Cuso4 Quora From quora.com
That s why we prefer to use zinc which is sacrificial to iron. Plating can be tricky but this method comes as close. The uses of copper plating. This will cause your steel parts to rust even faster. They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. This is called an immersion deposit and it usually has virtually no adhesion.
Iron plating onto copper base 2005.
This is probably the easiest way to create reliable attractive copper plate on many different metals. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. This will cause your steel parts to rust even faster. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. Plating can be tricky but this method comes as close. Iron plating onto copper base 2005.
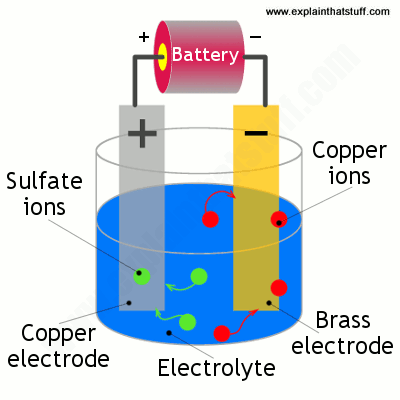 Source: explainthatstuff.com
Source: explainthatstuff.com
The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. Copper will create a galvanic reaction which is how most batteries work with the iron in the steel when your object is placed in water. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. Plating can be tricky but this method comes as close. This will cause your steel parts to rust even faster.
 Source: chemistryhive.com
Source: chemistryhive.com
Sir i ll make a soldering iron with copper base. They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. Iron plating onto copper base 2005. Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better. But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied.
 Source: m.youtube.com
Source: m.youtube.com
The lead must bond to the iron when it is dipped. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. Sir i ll make a soldering iron with copper base. They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. Plating can be tricky but this method comes as close.
Source:
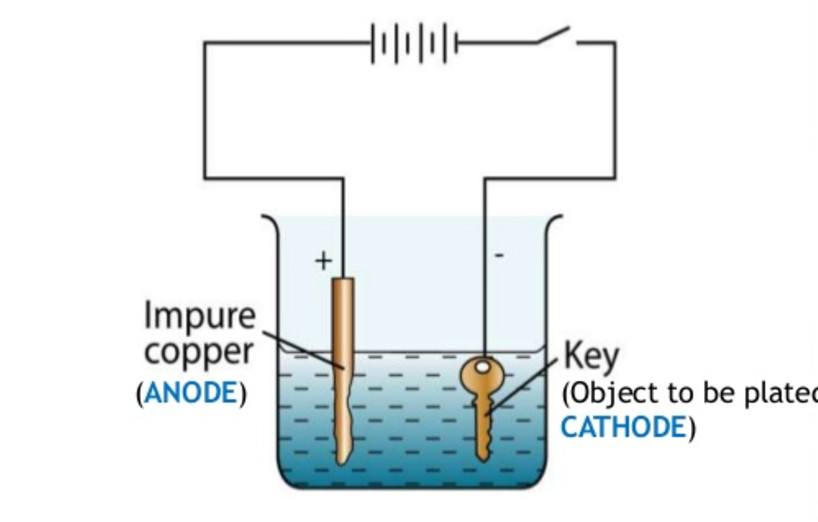
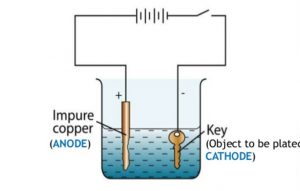
Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better. They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. An electrical circuit has now formed with the positive electrodes negative electrodes and an electrical current is flowing. 22 copper plating on iron michael corfield abstract t h e use of m o d e r n cleaning techniques has revealed a much wider use of copper alloy plating on iron than h a d previously b e e n recognized. We tried some iron plating but it won t work.
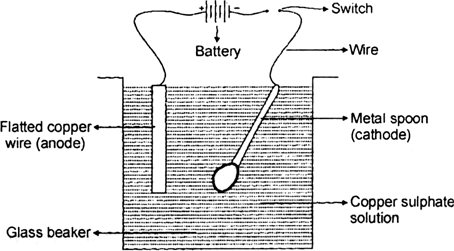 Source: zigya.com
Source: zigya.com
The uses of copper plating. Thank you very much. This p a p e r examines t h e evidence for plating t h e type of artefact on which it is found a n d t h e techniques used for plating. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. Plating can be tricky but this method comes as close.
 Source: classnotes.org.in
Source: classnotes.org.in
Copper plating material surfaces presents a number of advantages to substrates. An electrical circuit has now formed with the positive electrodes negative electrodes and an electrical current is flowing. Thank you very much. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. The lead must bond to the iron when it is dipped.
 Source: spark.iop.org
Source: spark.iop.org
They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. That s why we prefer to use zinc which is sacrificial to iron. The lead must bond to the iron when it is dipped. Copper plating material surfaces presents a number of advantages to substrates.
 Source: classnotes.org.in
Source: classnotes.org.in
The lead must bond to the iron when it is dipped. That s why we prefer to use zinc which is sacrificial to iron. They are next immersed in a second bath comprised of 10 parts nitric acid and 10 parts chloride of copper dissolved in 80 parts of hydrochloric acid specific gravity 1 1. Copper is only plated onto iron as the substrate for other plating such as nickel and chrome because it adheres better. This is probably the easiest way to create reliable attractive copper plate on many different metals.
 Source: indonesian.alibaba.com
Source: indonesian.alibaba.com
Copper plating material surfaces presents a number of advantages to substrates. Sir i ll make a soldering iron with copper base. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. This p a p e r examines t h e evidence for plating t h e type of artefact on which it is found a n d t h e techniques used for plating. This is called an immersion deposit and it usually has virtually no adhesion.
 Source: revision.co.zw
Source: revision.co.zw
22 copper plating on iron michael corfield abstract t h e use of m o d e r n cleaning techniques has revealed a much wider use of copper alloy plating on iron than h a d previously b e e n recognized. Placement of a metal such as an iron nail in the lemon bath containing suspended copper ions results in a simple copper plating of the iron nail. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. The uses of copper plating. The lead must bond to the iron when it is dipped.
 Source: sharrettsplating.com
Source: sharrettsplating.com
But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. Copper plating material surfaces presents a number of advantages to substrates. Because copper is a highly active metal it is not ideal for direct plating with iron unless a base coat of nickel is initially applied. The uses of copper plating. 22 copper plating on iron michael corfield abstract t h e use of m o d e r n cleaning techniques has revealed a much wider use of copper alloy plating on iron than h a d previously b e e n recognized.
 Source: revision.co.zw
Source: revision.co.zw
Plating can be tricky but this method comes as close. Thank you very much. The lead must bond to the iron when it is dipped. That s why we prefer to use zinc which is sacrificial to iron. Sir i ll make a soldering iron with copper base.
 Source: youtube.com
Source: youtube.com
Iron plating onto copper base 2005. The iron will rust away behind the copper plating which will flake off to expose more iron and the eventual corrosion will be worse than if there was no plating. Plating can be tricky but this method comes as close. Copper will create a galvanic reaction which is how most batteries work with the iron in the steel when your object is placed in water. The lead must bond to the iron when it is dipped.
 Source: yenka.com
Source: yenka.com
Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. This is probably the easiest way to create reliable attractive copper plate on many different metals. Ronald almazora fabrication co. While copper is a highly effective electroplating solution in many situations some specific properties of copper introduce certain limitations in its use. The uses of copper plating.
 Source: quora.com
Source: quora.com
But sorry your approach won t work for functional electroplating applications because copper is more noble than iron and will as you saw deposit on steel or cast iron without any current applied. Iron plating onto copper base 2005. Copper plating cast iron in the process of covering cast iron with a coating of copper the pieces of cast iron are first placed in a bath made of 50 parts of hydrochloric acid specific gravity 1 1 and one part of nitric acid. The lead must bond to the iron when it is dipped. An electrical circuit has now formed with the positive electrodes negative electrodes and an electrical current is flowing.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title copper plating iron by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.