Copper plating copper sulfate
Copper Plating Copper Sulfate. Dry it off on a paper towel. Copper sulfate cuso4 provides a source of copper ions. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Take a copper plate and clean it properly.
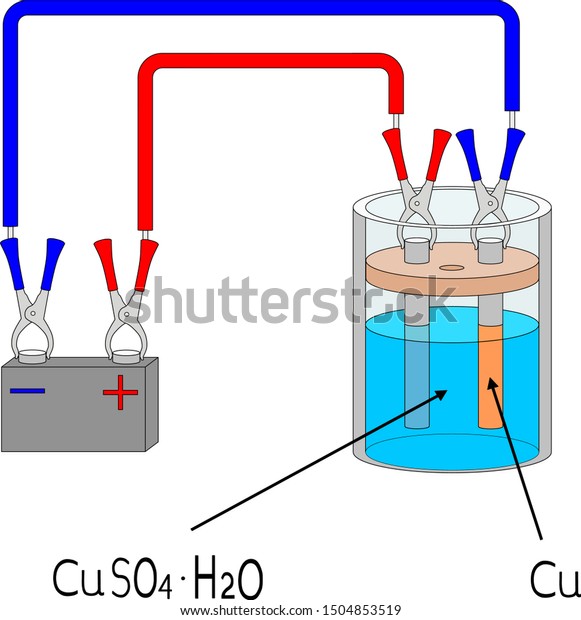 Electroplating Copper Copper Sulfate Electrolysis Stock Vector Royalty Free 1504853519 From shutterstock.com
Electroplating Copper Copper Sulfate Electrolysis Stock Vector Royalty Free 1504853519 From shutterstock.com
Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Copper sulfate cuso4 provides a source of copper ions. Your solution should be dark blue.
Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier.
Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. Take a copper plate and clean it properly. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Your solution should be dark blue. Copper sulfate cuso4 provides a source of copper ions. Take a nail and clean it properly.
 Source: chemedx.org
Source: chemedx.org
Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. The copper coating is an important component of.
 Source: docbrown.info
Source: docbrown.info
Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water.
 Source: shutterstock.com
Source: shutterstock.com
Stir copper sulfate into some hot water in a beaker until no more will dissolve. Your solution should be dark blue. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Copper sulfate cuso4 provides a source of copper ions.
 Source: docbrown.info
Source: docbrown.info
Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Connect the copper plate to the negative clip and nail to the positive clip.
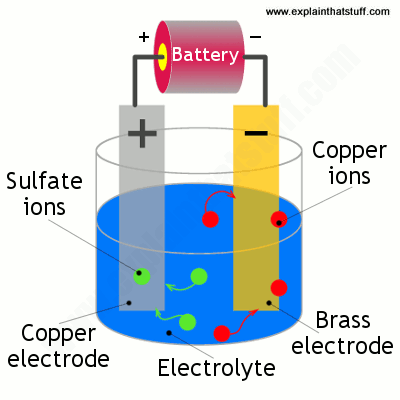 Source: explainthatstuff.com
Source: explainthatstuff.com
Copper sulfate cuso4 provides a source of copper ions. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Copper sulfate cuso4 provides a source of copper ions. The copper coating is an important component of. Dry it off on a paper towel.

Stir copper sulfate into some hot water in a beaker until no more will dissolve. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Connect the copper plate to the negative clip and nail to the positive clip. Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating.
 Source: rfcafe.com
Source: rfcafe.com
Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Dry it off on a paper towel. Take a nail and clean it properly. Connect the copper plate to the negative clip and nail to the positive clip. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas.
 Source: m.youtube.com
Source: m.youtube.com
Dry it off on a paper towel. Dry it off on a paper towel. Stir copper sulfate into some hot water in a beaker until no more will dissolve. Take a copper plate and clean it properly. The copper coating is an important component of.
 Source: quora.com
Source: quora.com
Connect the copper plate to the negative clip and nail to the positive clip. Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. Dry it off on a paper towel.
 Source: m.youtube.com
Source: m.youtube.com
Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Connect the copper plate to the negative clip and nail to the positive clip. Dry it off on a paper towel. Take a copper plate and clean it properly. Chloride ions cl combine with the organic species to form a complex that slows down plating rate on selective areas.
 Source: docbrown.info
Source: docbrown.info
Take a copper plate and clean it properly. Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Copper sulfate cuso4 provides a source of copper ions. Connect the copper plate to the negative clip and nail to the positive clip. Stir copper sulfate into some hot water in a beaker until no more will dissolve.
 Source: pfonline.com
Source: pfonline.com
Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Dry it off on a paper towel. Stir copper sulfate into some hot water in a beaker until no more will dissolve. The copper coating is an important component of. Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier.
 Source: docbrown.info
Source: docbrown.info
Sulfuric acid h2so4 makes the bath conductive and acts as a charge carrier. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water. Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. The copper coating is an important component of. Connect the copper plate to the negative clip and nail to the positive clip.
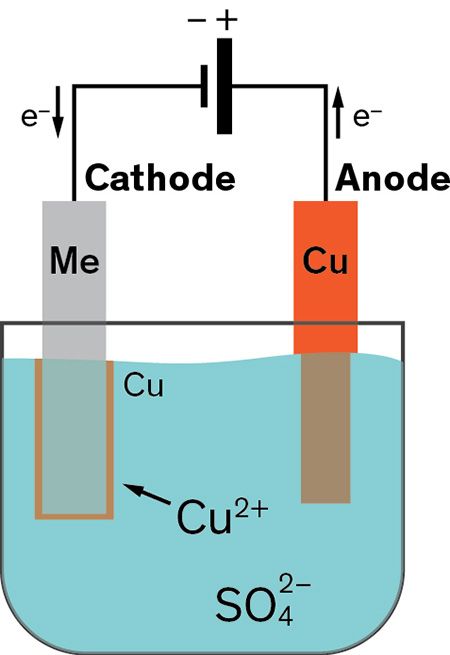
Copper sulfate cuso4 provides a source of copper ions. Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Dry it off on a paper towel. Connect the copper plate to the negative clip and nail to the positive clip. Take a copper plate and clean it properly.
 Source: docbrown.info
Source: docbrown.info
Electroplated copper is the most widely used preplating layer for improving the bond strength of the coating. The copper coating is an important component of. Take copper sulfate and pour 100 g of copper sulfate in water and mix it properly. Take a nail and clean it properly. Prepare the key for copper plating by cleaning it with a thin layer of toothpaste or soap and water.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title copper plating copper sulfate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





