Copper aluminum battery
Copper Aluminum Battery. The aluminium ion battery is conceptually similar to the lithium ion battery. However it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. This means that insertion of one aℓ is equivalent to three li displaystyle ce li ions in conventional intercalation cathodes. The farther two metals or alloys are separated on the table faster the corrosion of the less noble of the two will.
 Ultrafast Aluminum Battery Physicscentral From physicscentral.com
Ultrafast Aluminum Battery Physicscentral From physicscentral.com
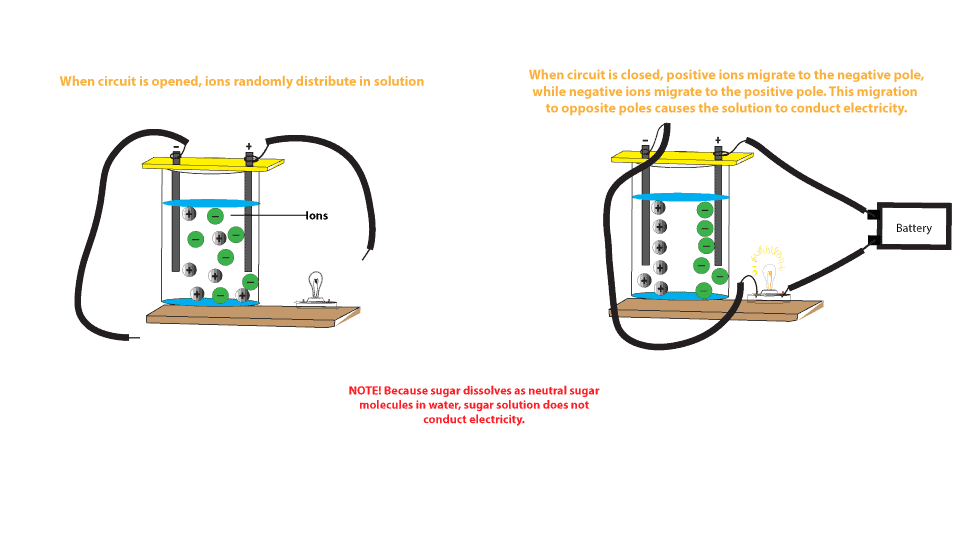
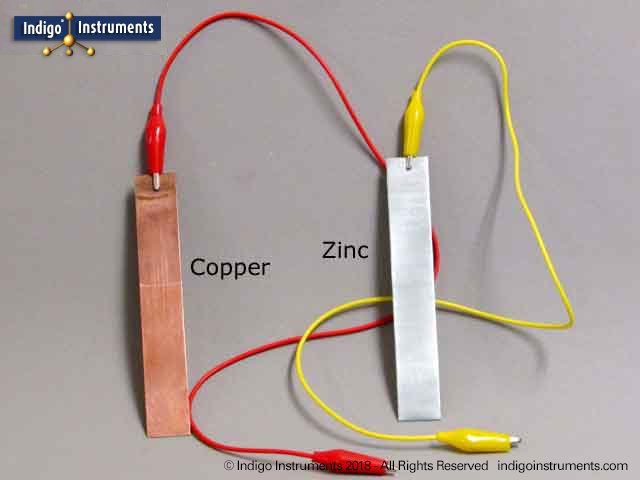
Really convenient for that aluminum copper battery here are of carefully classified collection. Accordingly the thermal conductivity of aluminum with 237 w mk approaches 59 that of copper hatch 1984 which also makes aluminum an excellent part of the battery to transfer or distribute the heat due to the charge or discharge of a battery. In general bolted connection of unplated aluminum to copper bus bars is discouraged. Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. When recharging aluminium ions return to the negative electrode and can exchange three electrons per ion. Figure 1 shows a battery constructed from a piece of thick copper grounding wire a strip of aluminum from a soda can and a glass of coke.
The farther two metals or alloys are separated on the table faster the corrosion of the less noble of the two will.
When the battery is discharged atoms from a metal anode are oxidised releasing electrons into the external. The majority of al to cu connections are made by applying silver or tin plating to the joint areas of either or both of the conductors. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. The presence of hydrogen sulfide h2s in the atmosphere is of main concern for base metal cu and silver plating. Aluminium air batteries are primary cells i e non rechargeable. The aluminium ion battery is conceptually similar to the lithium ion battery.
 Source: blog.indigoinstruments.com
Source: blog.indigoinstruments.com
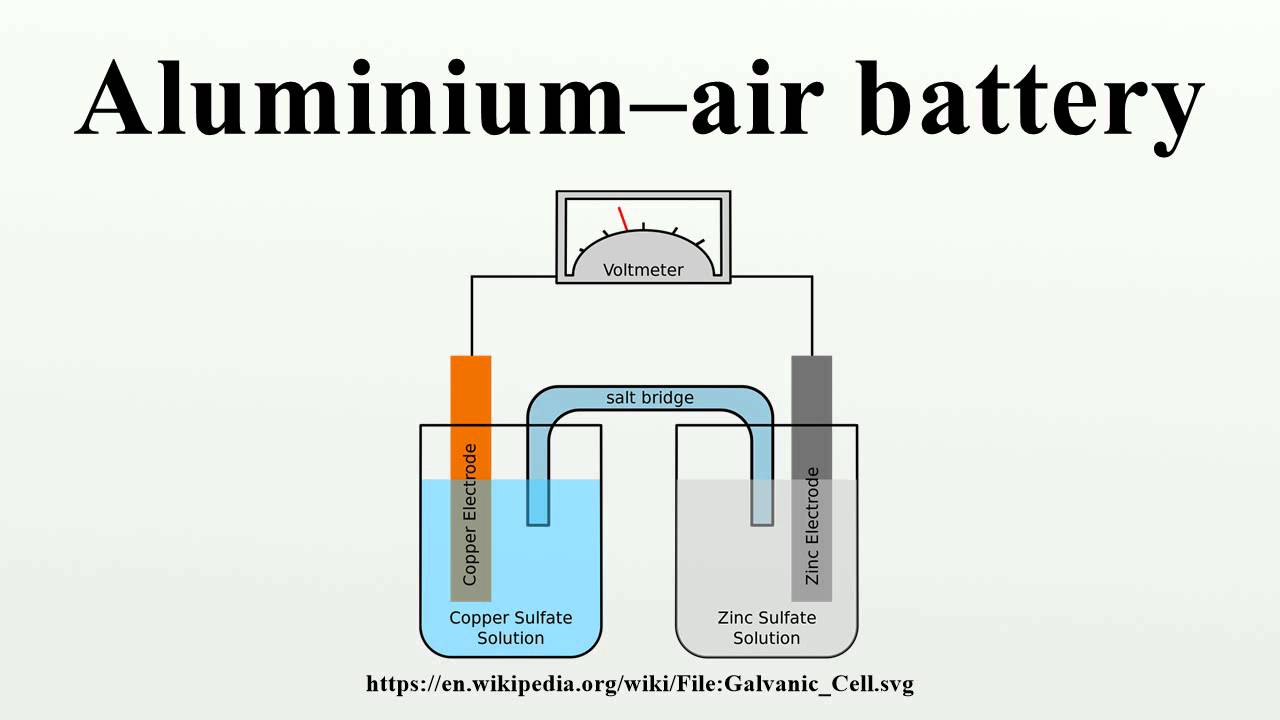
Aluminium air batteries are primary cells i e non rechargeable. This means that insertion of one aℓ is equivalent to three li displaystyle ce li ions in conventional intercalation cathodes. As the voltmeter shows this battery provides about 3 4 of a volt. In general bolted connection of unplated aluminum to copper bus bars is discouraged. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity.
 Source: splung.com
Source: splung.com
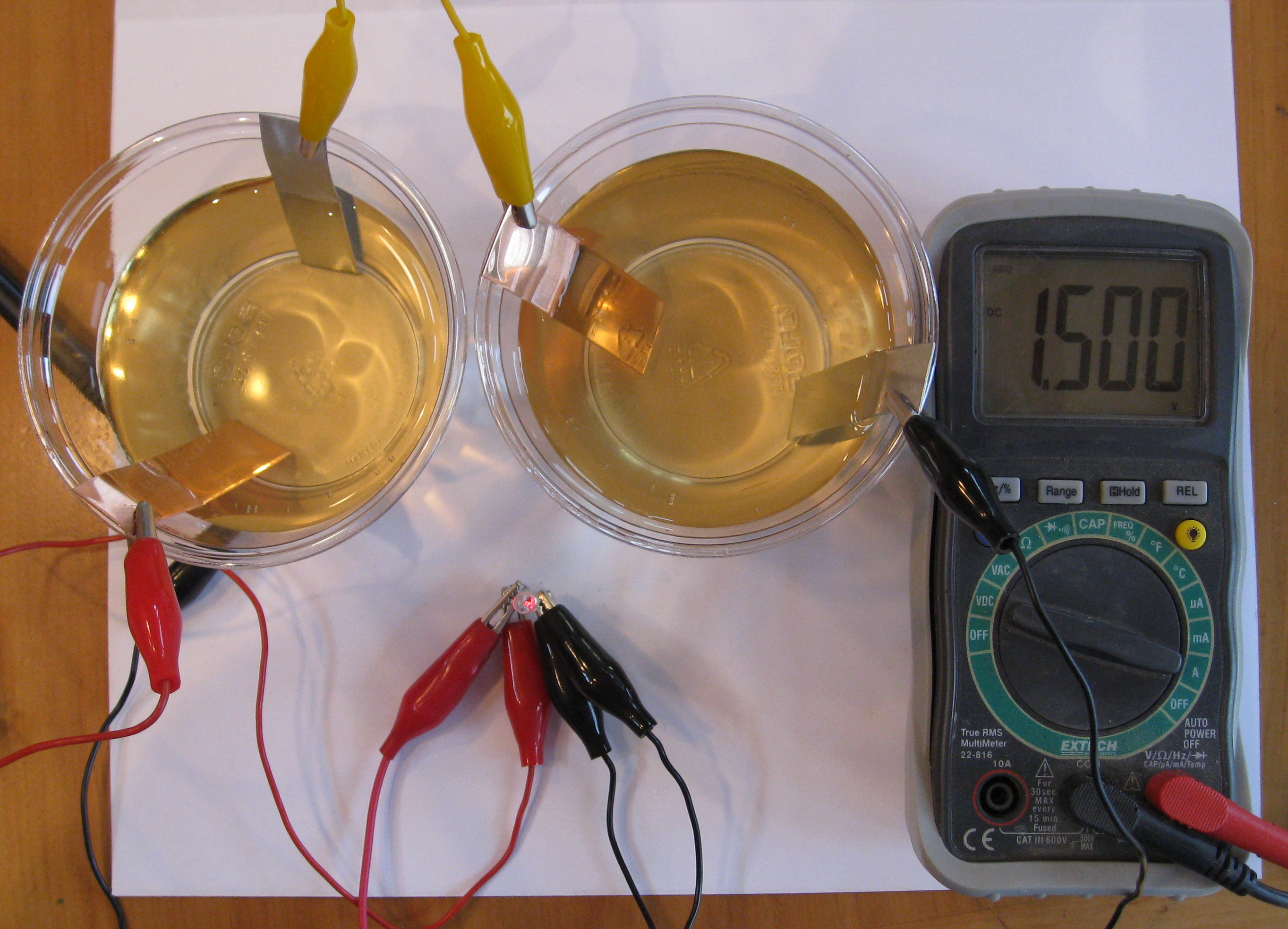
The aluminum was cut with a pair of tin snips and then sanded to remove the paint. The presence of hydrogen sulfide h2s in the atmosphere is of main concern for base metal cu and silver plating. Aluminium air batteries are primary cells i e non rechargeable. You can get every single series as easier as expected. In general bolted connection of unplated aluminum to copper bus bars is discouraged.
 Source: physicscentral.com
Source: physicscentral.com
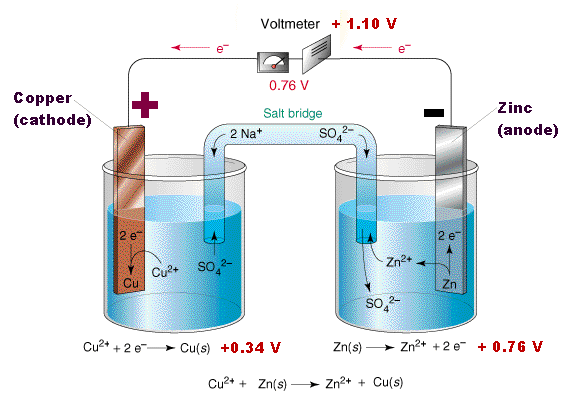
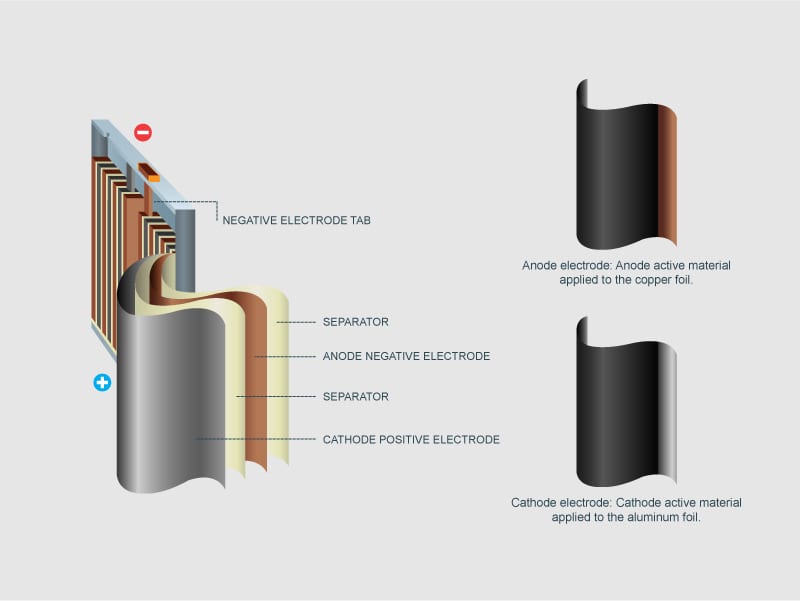
The majority of al to cu connections are made by applying silver or tin plating to the joint areas of either or both of the conductors. This means that insertion of one aℓ is equivalent to three li displaystyle ce li ions in conventional intercalation cathodes. Aluminium ion batteries are a class of rechargeable battery in which aluminium ions provide energy by flowing from the negative electrode of the battery the anode to the positive electrode the cathode. Aluminium air batteries are primary cells i e non rechargeable. When recharging aluminium ions return to the negative electrode and can exchange three electrons per ion.
 Source: sci-toys.com
Source: sci-toys.com
Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. This means that insertion of one aℓ is equivalent to three li displaystyle ce li ions in conventional intercalation cathodes. Figure 1 shows a battery constructed from a piece of thick copper grounding wire a strip of aluminum from a soda can and a glass of coke. However it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. When recharging aluminium ions return to the negative electrode and can exchange three electrons per ion.
 Source: physicscentral.com
Source: physicscentral.com
Some recent crude experiments to generate electricty from scrap metal to charge nicad batteries and capacitors to run led lamps and other low wattage items. The farther two metals or alloys are separated on the table faster the corrosion of the less noble of the two will. Accordingly the thermal conductivity of aluminum with 237 w mk approaches 59 that of copper hatch 1984 which also makes aluminum an excellent part of the battery to transfer or distribute the heat due to the charge or discharge of a battery. Thus since the ionic radii of aℓ. When the battery is discharged atoms from a metal anode are oxidised releasing electrons into the external.
 Source: youtube.com
Source: youtube.com
Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. The majority of al to cu connections are made by applying silver or tin plating to the joint areas of either or both of the conductors. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. Some recent crude experiments to generate electricty from scrap metal to charge nicad batteries and capacitors to run led lamps and other low wattage items. Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table.
 Source: cei.washington.edu
Source: cei.washington.edu
As the voltmeter shows this battery provides about 3 4 of a volt. Accordingly the thermal conductivity of aluminum with 237 w mk approaches 59 that of copper hatch 1984 which also makes aluminum an excellent part of the battery to transfer or distribute the heat due to the charge or discharge of a battery. Thus since the ionic radii of aℓ. The aluminum was cut with a pair of tin snips and then sanded to remove the paint. The majority of al to cu connections are made by applying silver or tin plating to the joint areas of either or both of the conductors.
 Source: chargedevs.com
Source: chargedevs.com
As the voltmeter shows this battery provides about 3 4 of a volt. The farther two metals or alloys are separated on the table faster the corrosion of the less noble of the two will. Aluminium ion batteries are a class of rechargeable battery in which aluminium ions provide energy by flowing from the negative electrode of the battery the anode to the positive electrode the cathode. Aluminum will be very susceptible to galvanic corrosion in contact with copper assuming that the two metals are also in contact with a common electrolyte such as water with some ionic content almost any text or handbook on corrosion will have galvanic series table. Thus since the ionic radii of aℓ.
 Source: youtube.com
Source: youtube.com
The aluminium ion battery is conceptually similar to the lithium ion battery. Thus since the ionic radii of aℓ. Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. Aluminium ion batteries are a class of rechargeable battery in which aluminium ions provide energy by flowing from the negative electrode of the battery the anode to the positive electrode the cathode.
 Source: sci-toys.com
Source: sci-toys.com
Accordingly the thermal conductivity of aluminum with 237 w mk approaches 59 that of copper hatch 1984 which also makes aluminum an excellent part of the battery to transfer or distribute the heat due to the charge or discharge of a battery. However it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. As the voltmeter shows this battery provides about 3 4 of a volt. The aluminium ion battery is conceptually similar to the lithium ion battery.
 Source: sci-toys.com
Source: sci-toys.com
As the voltmeter shows this battery provides about 3 4 of a volt. In general bolted connection of unplated aluminum to copper bus bars is discouraged. Aluminium air batteries are primary cells i e non rechargeable. Thus since the ionic radii of aℓ. The presence of hydrogen sulfide h2s in the atmosphere is of main concern for base metal cu and silver plating.
 Source: blog.indigoinstruments.com
Source: blog.indigoinstruments.com
Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. However it is possible to mechanically recharge the battery with new aluminium anodes made from recycling the hydrated aluminium oxide. The farther two metals or alloys are separated on the table faster the corrosion of the less noble of the two will. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. Accordingly the thermal conductivity of aluminum with 237 w mk approaches 59 that of copper hatch 1984 which also makes aluminum an excellent part of the battery to transfer or distribute the heat due to the charge or discharge of a battery.
 Source: targray.com
Source: targray.com
Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. Aluminium ion batteries are a class of rechargeable battery in which aluminium ions provide energy by flowing from the negative electrode of the battery the anode to the positive electrode the cathode. The majority of al to cu connections are made by applying silver or tin plating to the joint areas of either or both of the conductors. When recharging aluminium ions return to the negative electrode and can exchange three electrons per ion. You can get every single series as easier as expected.
 Source: youtube.com
Source: youtube.com
This means that insertion of one aℓ is equivalent to three li displaystyle ce li ions in conventional intercalation cathodes. The presence of hydrogen sulfide h2s in the atmosphere is of main concern for base metal cu and silver plating. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. You can get every single series as easier as expected. Really convenient for that aluminum copper battery here are of carefully classified collection.
 Source: aluminium-flat-bar.com
Source: aluminium-flat-bar.com
Moreover what have been left to consumers are of course unparalleled quality with low price as well as adorable discount. Once the aluminium anode is consumed by its reaction with atmospheric oxygen at a cathode immersed in a water based electrolyte to form hydrated aluminium oxide the battery will no longer produce electricity. You can get every single series as easier as expected. The presence of hydrogen sulfide h2s in the atmosphere is of main concern for base metal cu and silver plating. As the voltmeter shows this battery provides about 3 4 of a volt.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title copper aluminum battery by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.