Colours of metal oxides
Colours Of Metal Oxides. Not too strong effect. It is a component of glass. White to pale yellow. The basic structural unit of fe mn al and ti oxides are cationic metal centers bound to six oxygens.
 Richard Nakka S Experimental Rocketry Site From nakka-rocketry.net
Richard Nakka S Experimental Rocketry Site From nakka-rocketry.net
Not too strong effect. Iron ii oxide black used as pigment. Sodium oxide na 2 o is a white solid that melts at 1132 c and decomposes at 1950 c. With chlorine donors yields green color without chlorine burns white. It melts at 1570 c. Chromium vi oxide red toxic oxidizing agent.
Iron iii oxide rust reddish.
Magnesium oxide mgo solid white powder. Many transition metal oxides are black but their underlying structure is different. Exothermic carbon glows orange. Highly exothermic magnesium burns with bright white flame. Cobalt ii iii oxide black. In spite of being a metallic oxide it has acidic properties in its hydrated form.
 Source: researchgate.net
Source: researchgate.net
Cobalt ii oxide dark green or black. Cucl the richest blue flame. Exothermic carbon glows orange. Not too strong effect. Magnesium oxide mgo solid white powder.
 Source: compoundchem.com
Source: compoundchem.com
The basic structural unit of fe mn al and ti oxides are cationic metal centers bound to six oxygens. Rubidium oxide rb 2 o is a yellow solid that melts at 500 c. With chlorine donors yields green color without chlorine burns white. Cao calcium oxide quicklime. In green compositions usually used with perchlorates.
 Source: chemistryscl.com
Source: chemistryscl.com
It is a component of glass. Magnesium oxide mgo solid white powder. Iron iii oxide rust reddish. Mno manganese ii oxide manganese monoxide. Not too strong effect.
 Source: scielo.org.co
Source: scielo.org.co
Cuo copper ii oxide cupric oxide. If one drastically reduces the size of the oxygens in relation to the metal cations this arrangement can be visualized as an octahedron figure 1 the octahedra may be linked to each other in three ways sharing oxygen corners 1 o sharing edges 2 o or faces 3 o. Iron ii oxide black used as pigment. With chlorine donors yields green color without chlorine burns white. Being gold a noble metal which means that it is oxidized with difficulty this is the most stable gold oxide.
 Source: aerogel.org
Source: aerogel.org
Being gold a noble metal which means that it is oxidized with difficulty this is the most stable gold oxide. Magnesium oxide mgo solid white powder. Cobalt ii oxide dark green or black. Learn vocabulary terms and more with flashcards games and other study tools. Cobalt iii oxide black.
 Source: researchgate.net
Source: researchgate.net
Highly exothermic magnesium burns with bright white flame. K2o2 potassium oxide. Cobalt iii oxide black. Highly exothermic magnesium burns with bright white flame. Bac 2 o 4.
 Source: art-paints.com
Source: art-paints.com
Learn vocabulary terms and more with flashcards games and other study tools. Magnesium oxide mgo solid white powder. Copper i oxide reddish. In spite of being a metallic oxide it has acidic properties in its hydrated form. Lithium oxide li 2 o is the lightest alkali metal oxide and a white solid.
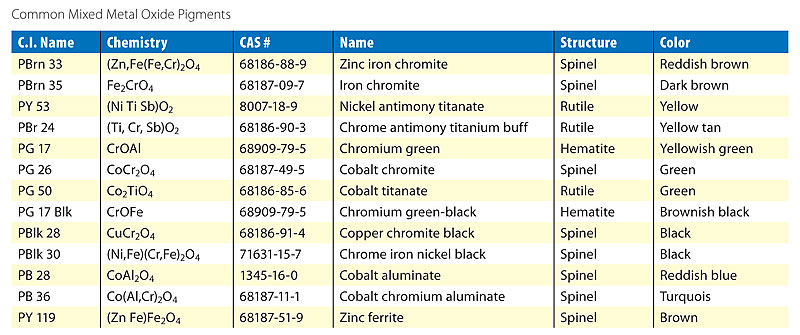 Source: pcimag.com
Source: pcimag.com
Cao calcium oxide quicklime. Rubidium oxide rb 2 o is a yellow solid that melts at 500 c. Copper i oxide reddish. Mno manganese ii oxide manganese monoxide. Cobalt ii oxide dark green or black.
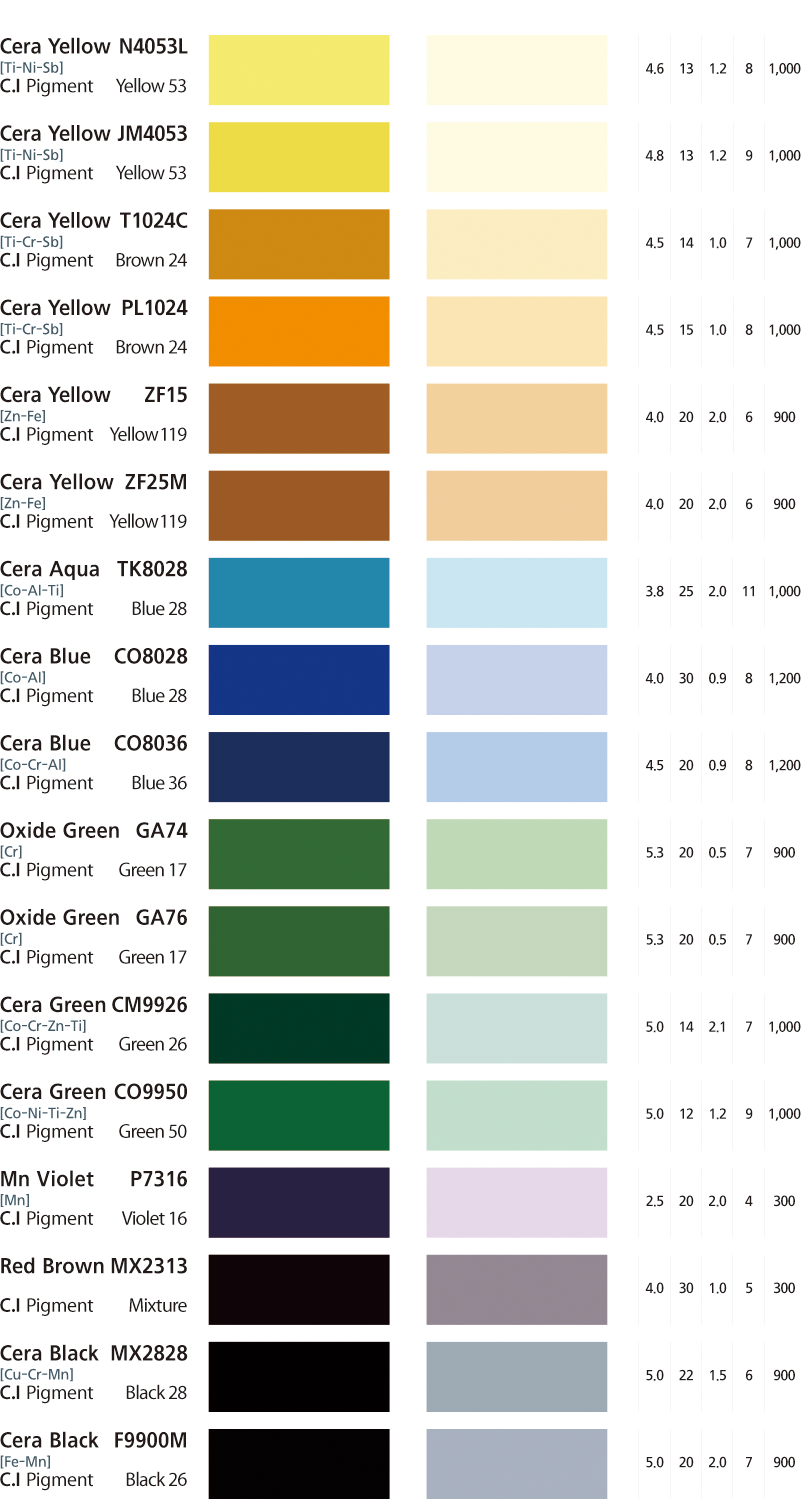 Source: m.cccworld.co.kr
Source: m.cccworld.co.kr
Potassium oxide k 2 o is a pale yellow solid that decomposes at 350 c. Sodium oxide na 2 o is a white solid that melts at 1132 c and decomposes at 1950 c. Copper ii oxide black. Cobalt ii iii oxide black. Cucl the richest blue flame.
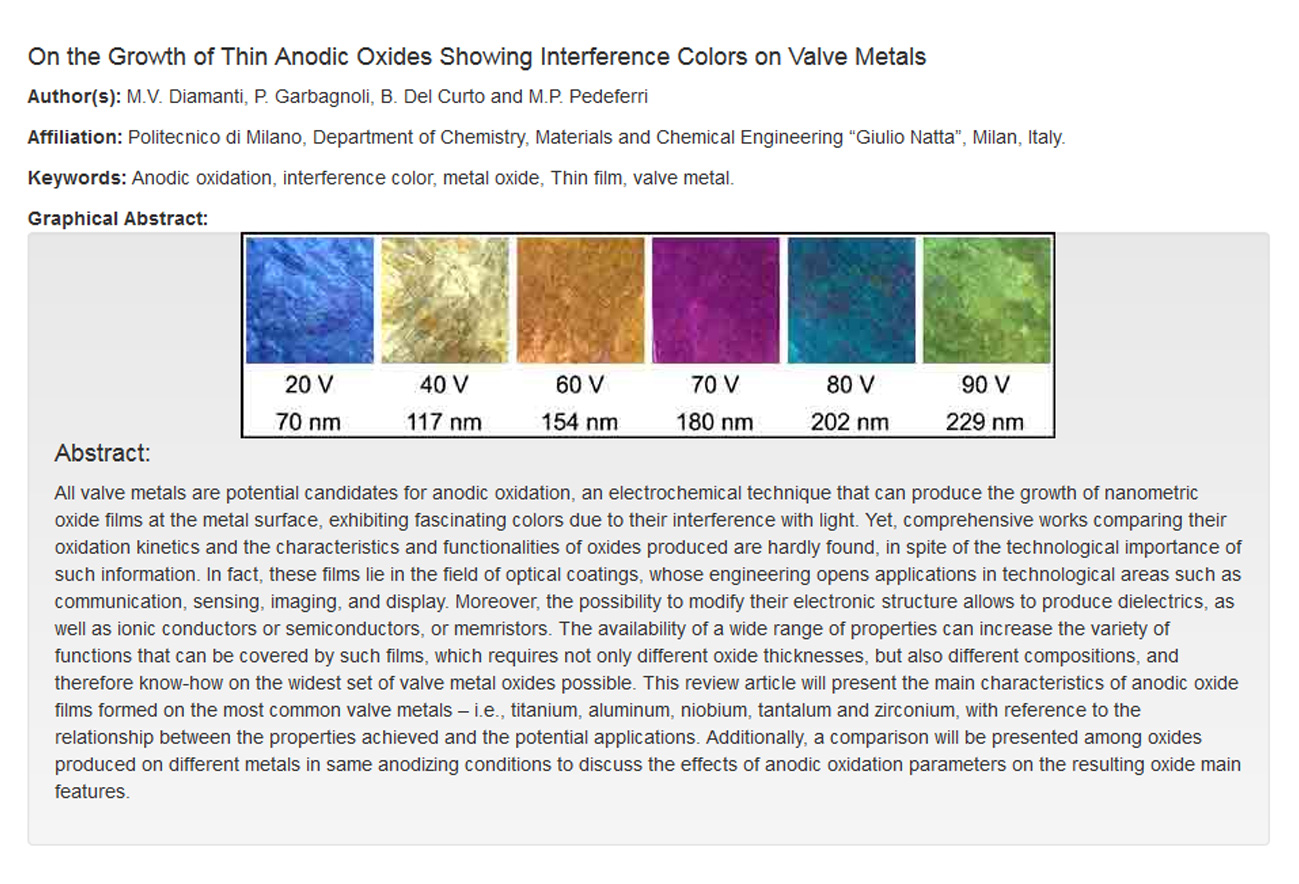 Source: tifun.chem.polimi.it
Source: tifun.chem.polimi.it
Iron iii oxide rust reddish. A pretty color when ammonium perchlorate is used as oxidizer. Copper ii oxide black. Learn vocabulary terms and more with flashcards games and other study tools. Many transition metal oxides are black but their underlying structure is different.
 Source: nakka-rocketry.net
Source: nakka-rocketry.net
Start studying colours of common metals metal oxides metal hydroxides and carbon. Cucl the richest blue flame. Cobalt iii oxide black. Exothermic carbon glows orange. Sodium oxide na 2 o is a white solid that melts at 1132 c and decomposes at 1950 c.
 Source: compoundchem.com
Source: compoundchem.com
It melts at 1570 c. Cobalt ii iii oxide black. Cuo copper ii oxide cupric oxide. Magnesium oxide mgo solid white powder. In spite of being a metallic oxide it has acidic properties in its hydrated form.
 Source: curriculumvisions.com
Source: curriculumvisions.com
I know of no websites with this information. Cobalt iii oxide black. In green compositions usually used with perchlorates. It implies a red color to the glass and is used for antifouling paints albert wilbur schlechten 2017. Copper ii oxide black.
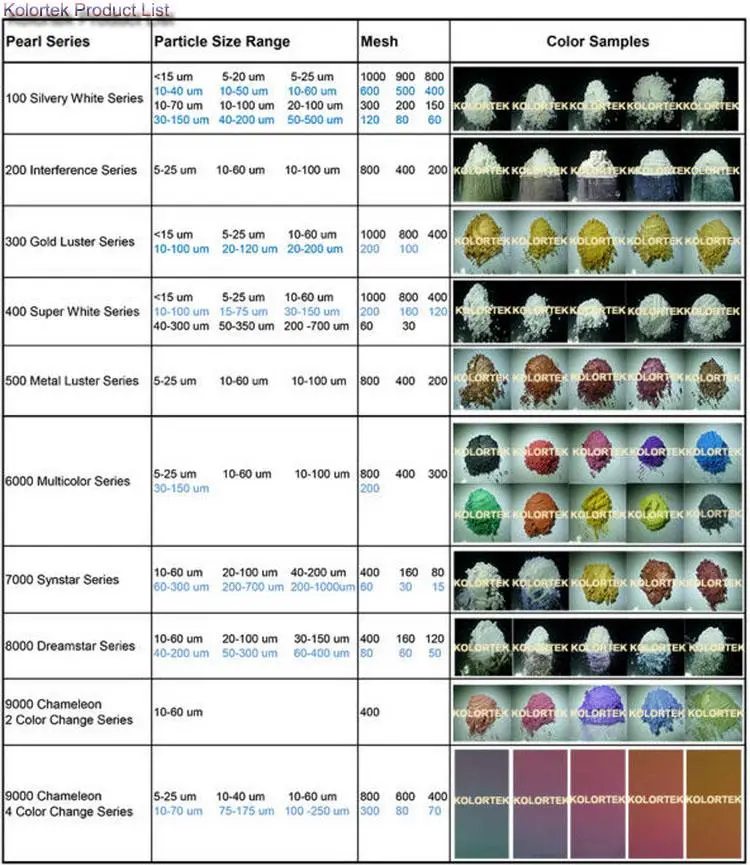 Source: alibaba.com
Source: alibaba.com
Cucl the richest blue flame. Chromium vi oxide red toxic oxidizing agent. Highly exothermic magnesium burns with bright white flame. Iron ii oxide black used as pigment. We would like to show you a description here but the site won t allow us.
 Source: techmen.com.sg
Source: techmen.com.sg
Copper ii oxide black. It implies a red color to the glass and is used for antifouling paints albert wilbur schlechten 2017. Many transition metal oxides are black but their underlying structure is different. Sodium oxide na 2 o is a white solid that melts at 1132 c and decomposes at 1950 c. With chlorine donors yields green color without chlorine burns white.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title colours of metal oxides by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.