Characteristics of metal and nonmetal
Characteristics Of Metal And Nonmetal. Graphite which is good conducter of electric. Properties of metals and nonmetals properties of elements distinguish them into metals and nonmetals. Metals form oxides that are basic but non metals form oxides that are acidic. Typical nonmetals have a dull coloured or colourless appearance.
 Icse Grade 10 Chemistry Metallurgy Lessons Exercises And Practice Tests Chemistry Classroom Gcse Science Chemistry From pinterest.com
Icse Grade 10 Chemistry Metallurgy Lessons Exercises And Practice Tests Chemistry Classroom Gcse Science Chemistry From pinterest.com
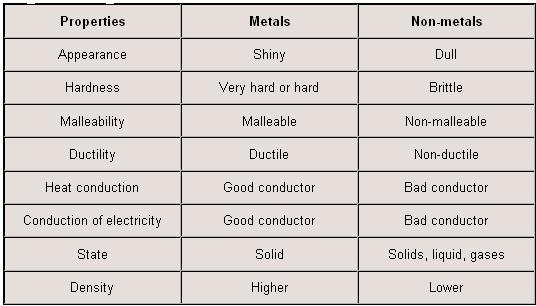
Metals conduct heat well because they can absorb a lot of kinetic energy without breaking their bonds. Elements that are nonmetals are hydrogen carbon nitrogen phosphorus oxygen sulfur selenium all of the halogens and the noble gases. Matt meadows getty images nonmetals. The reason why nonmetals are poor conductors is related to the reason why metals are comparatively good conductors. 2 non metals are brittle exception. Nonmetals with the exception of hydrogen are located on the right side of the periodic table.
Some chemical properties of non metals are listed below.
Unlike metals nonmetals aren t malleable and ductile. These elements are shown in the following figure. 3 they are bad conductor of heat and electricity. Graphite which is good conducter of electric. Some physical properties of non metals are listed below. And have acidic oxides.
 Source: socratic.org
Source: socratic.org
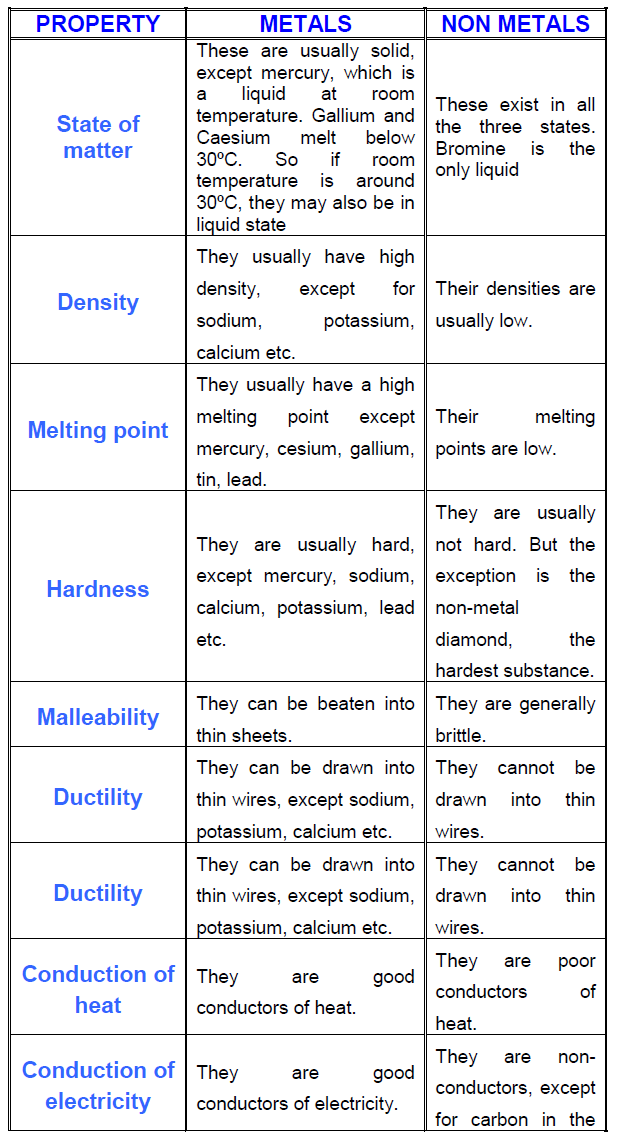
These elements are shown in the following figure. And have acidic oxides. The nonmetals in the periodic table. Some chemical properties of non metals are listed below. Typical nonmetals have a dull coloured or colourless appearance.

Usually nonmetals react with other nonmetals in high temperature. Typical nonmetals have a dull coloured or colourless appearance. The characters of non metal are as follows. Metalloids are metallic looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. 2 non metals are brittle exception.
 Source: researchgate.net
Source: researchgate.net
Most nonmetals do not react with air in room temperature. Properties of metals and nonmetals properties of elements distinguish them into metals and nonmetals. 2 non metals are brittle exception. They react with oxygen to form sulfur dioxide and carbon dioxide. 1 non metals are non luster.
 Source: studylib.net
Source: studylib.net
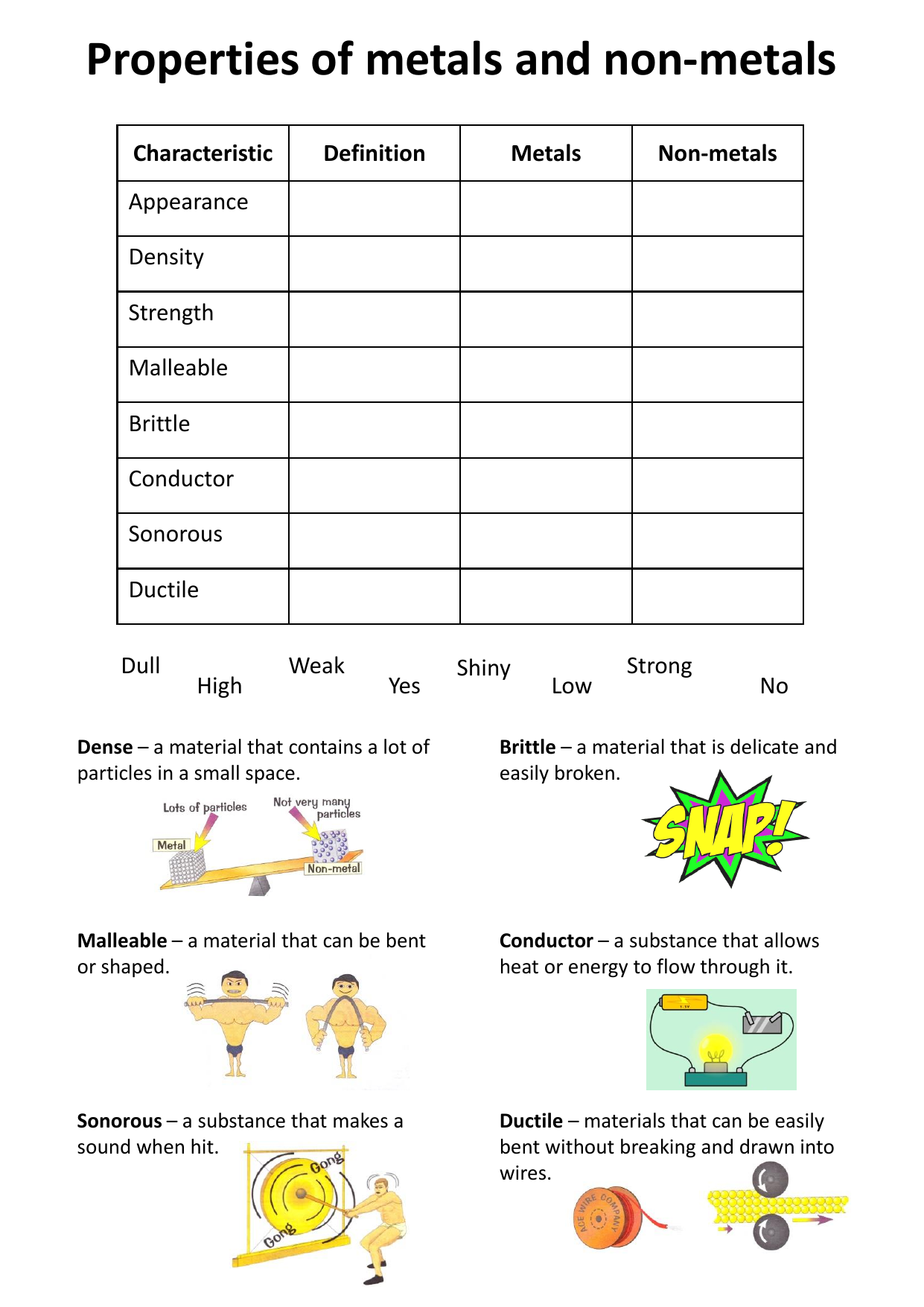
Some physical properties of non metals are listed below. Properties of non metals physical properties of non metals. These are not sonorous. The characters of non metal are as follows. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions.

These elements are shown in the following figure. Metals form oxides that are basic but non metals form oxides that are acidic. While nonmetals are those elements which are not malleable ductile sonorous and are poor conductors of heat and electricity. Unlike metals nonmetals aren t malleable and ductile. Some nonmetals are liquids.
 Source: thoughtco.com
Source: thoughtco.com
2 non metals are brittle exception. Poor conductors of electricity and heat. Characteristics of non metals 1 non metals are not malleable. And have acidic oxides. Diamond 3 non metals are bad conducter of heat and electricity exception.
 Source: sciencenotes.org
Source: sciencenotes.org
2 non metals are not ductile. Properties of metals and nonmetals properties of elements distinguish them into metals and nonmetals. Maybe solids liquids or gases at room temperature. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions. Unlike metals nonmetals aren t malleable and ductile.
Source: chemistryadda.blogspot.com
Metalloids are metallic looking brittle solids that are either semiconductors or exist in semiconducting forms and have amphoteric or weakly acidic oxides. Typical nonmetals have a dull coloured or colourless appearance. For example sulfur and carbon are both non metals. These elements are shown in the following figure. And have acidic oxides.
 Source: pinterest.com.mx
Source: pinterest.com.mx
Are poor conductors of heat and electricity. Chemical properties of non metals. For example sulfur and carbon are both non metals. Nonmetals have properties opposite those of the metals. Most nonmetals do not react with air in room temperature.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
Some chemical properties of non metals are listed below. Usually nonmetals react with other nonmetals in high temperature. The characters of non metal are as follows. 3 they are bad conductor of heat and electricity. Some physical properties of non metals are listed below.
 Source: aplustopper.com
Source: aplustopper.com
Some nonmetals are liquids. Characteristics of non metals 1 non metals are not malleable. These are not sonorous. The nonmetals in the periodic table. Nonmetals with the exception of hydrogen are located on the right side of the periodic table.
 Source: pinterest.com
Source: pinterest.com
They react with oxygen to form sulfur dioxide and carbon dioxide. Nonmetals in contrast need a fixed arrangement of atoms to remain stable so too much kinetic energy will overcome their bonds. Most nonmetals do not react with air in room temperature. The characters of non metal are as follows. While nonmetals are those elements which are not malleable ductile sonorous and are poor conductors of heat and electricity.
 Source: thoughtco.com
Source: thoughtco.com
Characteristics of non metals 1 non metals are not malleable. Diamond 3 non metals are bad conducter of heat and electricity exception. Usually nonmetals react with other nonmetals in high temperature. Some physical properties of non metals are listed below. Chemical properties of non metals.
 Source: secondaryscience4all.wordpress.com
Source: secondaryscience4all.wordpress.com
Poor conductors of electricity and heat. Some nonmetals are liquids. Nonmetals react more with metals than with nonmetals. Nonmetals also tend to be relatively poor conductors of heat and electricity though some exceptions exist. The characters of non metal are as follows.
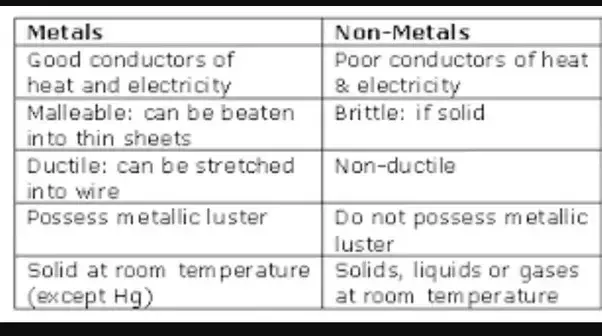 Source: quora.com
Source: quora.com
Chemical properties of non metals. Matt meadows getty images nonmetals. 1 non metals are non luster. Properties of non metals physical properties of non metals. Diamond 3 non metals are bad conducter of heat and electricity exception.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title characteristics of metal and nonmetal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.