Causes of corrosion in metals
Causes Of Corrosion In Metals. This potential difference causes the flow of electrons within the cell. This very common form of corrosion attacks the entire surface of a metal structure. Causes of galvanic corrosion every galvanic cell functions due to potential difference. And some metals do not corrode at all such as palladium silver platinum and gold.
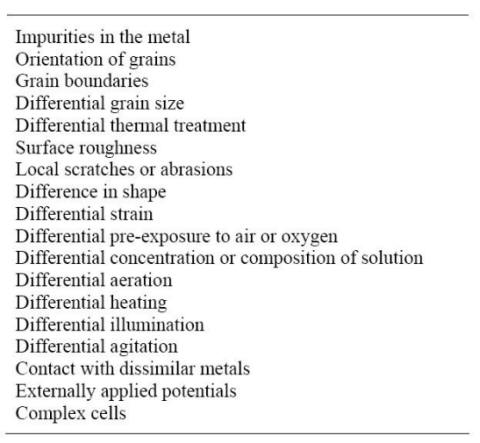 Corrosion Cells And Factors Causing Corrosion From corrosion-doctors.org
Corrosion Cells And Factors Causing Corrosion From corrosion-doctors.org
Characteristics of the metal pipe and electrical currents are common causes of corrosion. The ability of the metal electrode to lose electrons and to become oxidized is known as oxidation potential. I contact between unprotected dissimilar metals where moisture is present. This type of corrosion commonly seen in metal tubes carrying moving fluids in it. Metal corrosion will also occur by a chemical reaction with gaseous substances like acid vapors ammonia gas and gases containing sulpher. Erosion corrosion is caused by mechanical abrasion due to the relative movement between metal surfaces and corrosive fluids.
Characteristics of the metal pipe and electrical currents are common causes of corrosion.
Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment. This potential difference causes the flow of electrons within the cell. The world corrosion organization estimates the global cost of corrosion to be about us 2 5 trillion annually and a large portion of this as much as 25 could be eliminated by applying simple well understood prevention techniques. As a result it is possible to plan for and manage general attack corrosion. In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. It is defined as the natural process that causes the transformation of pure metals to undesirable substances when they react with substances like water or air.
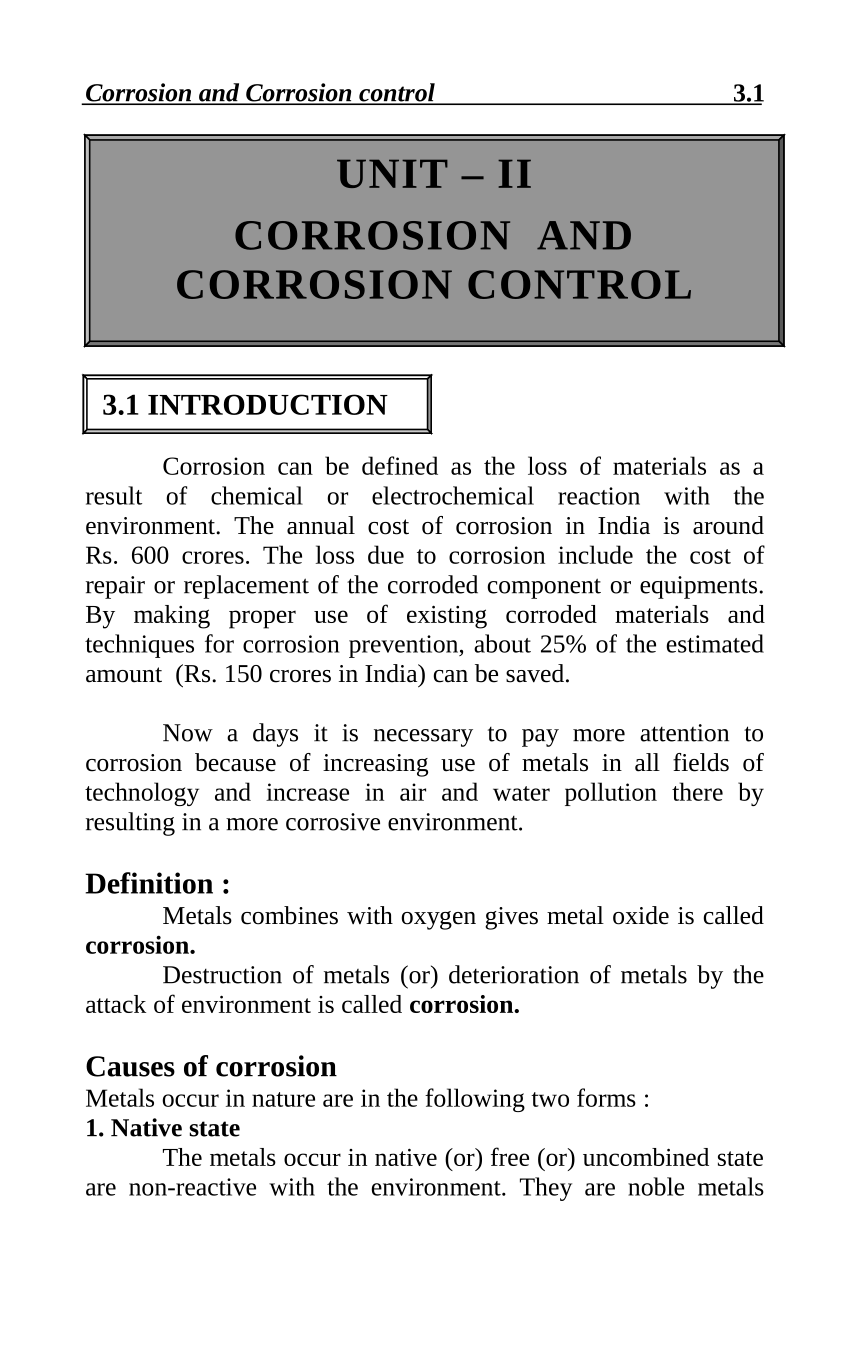 Source: researchgate.net
Source: researchgate.net
Metals higher on the galvanic series tend to be more corrosive while metals further apart on the series are more likely to cause galvanic corrosion. Metals higher on the galvanic series tend to be more corrosive while metals further apart on the series are more likely to cause galvanic corrosion. Erosion corrosion is caused by mechanical abrasion due to the relative movement between metal surfaces and corrosive fluids. It is caused by chemical or electrochemical reactions. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment.
 Source: learnpick.in
Source: learnpick.in
It is caused by chemical or electrochemical reactions. Characteristics of the metal pipe and electrical currents are common causes of corrosion. In galvanic corrosion the size of the cathode in relation to the anode has a large influence on corrosion as well. Factors other than water characteristics and bacteria can also influence corrosion. Metal corrosion will also occur by a chemical reaction with gaseous substances like acid vapors ammonia gas and gases containing sulpher.
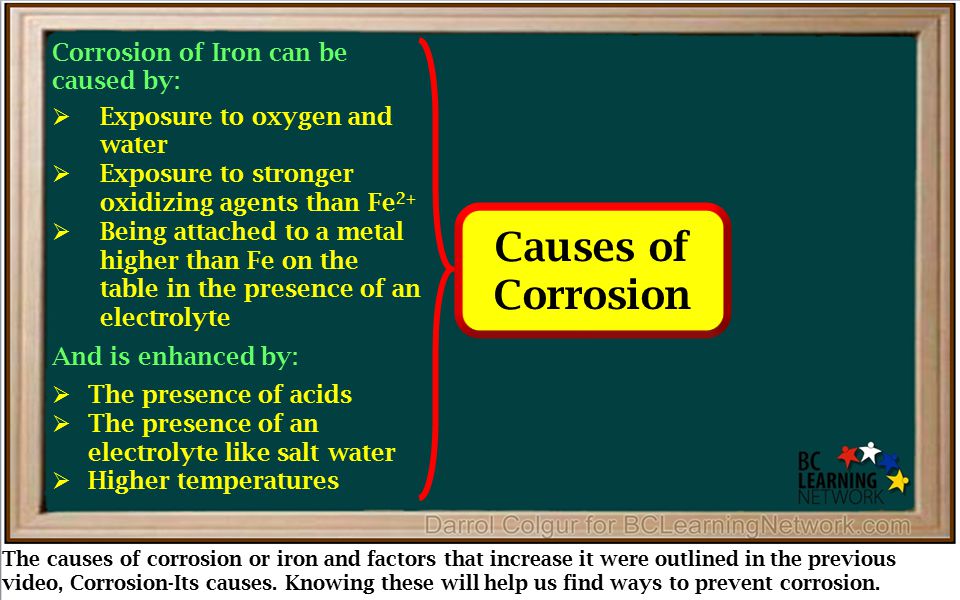 Source: slideplayer.com
Source: slideplayer.com
Without exception the corrosion of metals and alloys majority of materials used in industry in aqueous environments the most often encountered environment is an electrochemical reaction. Factors other than water characteristics and bacteria can also influence corrosion. Rust is the result of corroding steel after the iron fe particles have been exposed to oxygen and moisture e g humidity vapor immersion. In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. Characteristics of the metal pipe and electrical currents are common causes of corrosion.
 Source: galvanizing.org.uk
Source: galvanizing.org.uk
Without exception the corrosion of metals and alloys majority of materials used in industry in aqueous environments the most often encountered environment is an electrochemical reaction. The iron particles then become oxidized which results in the formation of fe. Erosion corrosion is caused by mechanical abrasion due to the relative movement between metal surfaces and corrosive fluids. And some metals do not corrode at all such as palladium silver platinum and gold. It is defined as the natural process that causes the transformation of pure metals to undesirable substances when they react with substances like water or air.
 Source: slideshare.net
Source: slideshare.net
In galvanic corrosion the size of the cathode in relation to the anode has a large influence on corrosion as well. The iron particles then become oxidized which results in the formation of fe. Rust is the result of corroding steel after the iron fe particles have been exposed to oxygen and moisture e g humidity vapor immersion. This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment.
 Source: slideplayer.com
Source: slideplayer.com
Factors other than water characteristics and bacteria can also influence corrosion. This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal. As a result it is possible to plan for and manage general attack corrosion. Factors other than water characteristics and bacteria can also influence corrosion. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment.
 Source: rai-technical-solutions.com
Source: rai-technical-solutions.com
Corrosion particularly signifies the process that is related to the weakening or degradation of the metal parts and the processes are generally electrochemical in nature. It is defined as the natural process that causes the transformation of pure metals to undesirable substances when they react with substances like water or air. And some metals do not corrode at all such as palladium silver platinum and gold. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment. Rust is the result of corroding steel after the iron fe particles have been exposed to oxygen and moisture e g humidity vapor immersion.
 Source: xapps.xyleminc.com
Source: xapps.xyleminc.com
The world corrosion organization estimates the global cost of corrosion to be about us 2 5 trillion annually and a large portion of this as much as 25 could be eliminated by applying simple well understood prevention techniques. Without exception the corrosion of metals and alloys majority of materials used in industry in aqueous environments the most often encountered environment is an electrochemical reaction. As a result it is possible to plan for and manage general attack corrosion. When steel is exposed to water the iron particles are lost to the water s acidic electrolytes. The iron particles then become oxidized which results in the formation of fe.
 Source: corrosion-doctors.org
Source: corrosion-doctors.org
In galvanic corrosion the size of the cathode in relation to the anode has a large influence on corrosion as well. In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. Erosion corrosion is caused by mechanical abrasion due to the relative movement between metal surfaces and corrosive fluids. When steel is exposed to water the iron particles are lost to the water s acidic electrolytes. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment.
 Source: slideshare.net
Source: slideshare.net
As a result it is possible to plan for and manage general attack corrosion. In galvanic corrosion the size of the cathode in relation to the anode has a large influence on corrosion as well. When steel is exposed to water the iron particles are lost to the water s acidic electrolytes. Factors other than water characteristics and bacteria can also influence corrosion. This type of corrosion commonly seen in metal tubes carrying moving fluids in it.
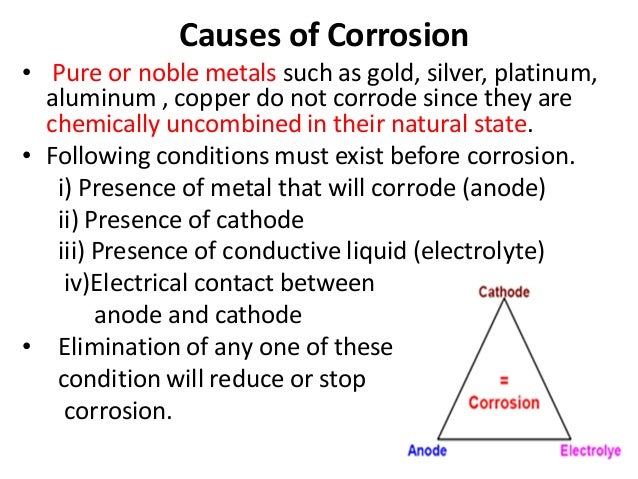 Source: slideshare.net
Source: slideshare.net
Without exception the corrosion of metals and alloys majority of materials used in industry in aqueous environments the most often encountered environment is an electrochemical reaction. This very common form of corrosion attacks the entire surface of a metal structure. In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. The ability of the metal electrode to lose electrons and to become oxidized is known as oxidation potential. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment.
 Source: tuf-bar.com
Source: tuf-bar.com
The iron particles then become oxidized which results in the formation of fe. Factors other than water characteristics and bacteria can also influence corrosion. Without exception the corrosion of metals and alloys majority of materials used in industry in aqueous environments the most often encountered environment is an electrochemical reaction. In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal.
 Source: slideplayer.com
Source: slideplayer.com
This very common form of corrosion attacks the entire surface of a metal structure. Metals higher on the galvanic series tend to be more corrosive while metals further apart on the series are more likely to cause galvanic corrosion. This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal. When steel is exposed to water the iron particles are lost to the water s acidic electrolytes. Metal corrosion will also occur by a chemical reaction with gaseous substances like acid vapors ammonia gas and gases containing sulpher.
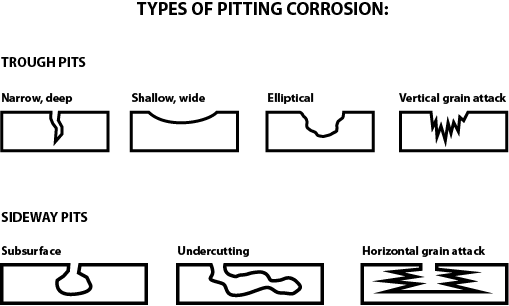 Source: gibsonstainless.com
Source: gibsonstainless.com
The iron particles then become oxidized which results in the formation of fe. When steel is exposed to water the iron particles are lost to the water s acidic electrolytes. The world corrosion organization estimates the global cost of corrosion to be about us 2 5 trillion annually and a large portion of this as much as 25 could be eliminated by applying simple well understood prevention techniques. This reaction causes damage and disintegration of the metal starting from the portion of the metal exposed to the environment and spreading to the entire bulk of the metal. Factors other than water characteristics and bacteria can also influence corrosion.
 Source: ddcoatings.co.uk
Source: ddcoatings.co.uk
In this case the surface of metal gets deteriorated gradually by the abrasion of fast moving fluids and cavities are also formed. The iron particles then become oxidized which results in the formation of fe. This very common form of corrosion attacks the entire surface of a metal structure. Corrosion can be defined in a more simplistic way as deterioration of materials under the influence of an environment. While general attack corrosion can cause a metal to fail it is also a known and predictable issue.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title causes of corrosion in metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





