Can salt conduct electricity
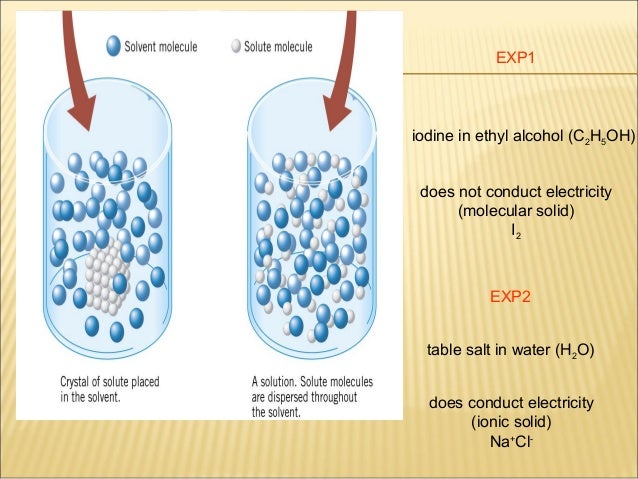
Can Salt Conduct Electricity. That means ions become seperated and can move easily in solution phase or molten phase. Electricity is a steady flow of electrons or electrically charged particles through a substance. But salt in aqueous or in molten state has movable ions. So solid salts cannot conduct electricity.
 Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T From masterconceptsinchemistry.com
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T From masterconceptsinchemistry.com
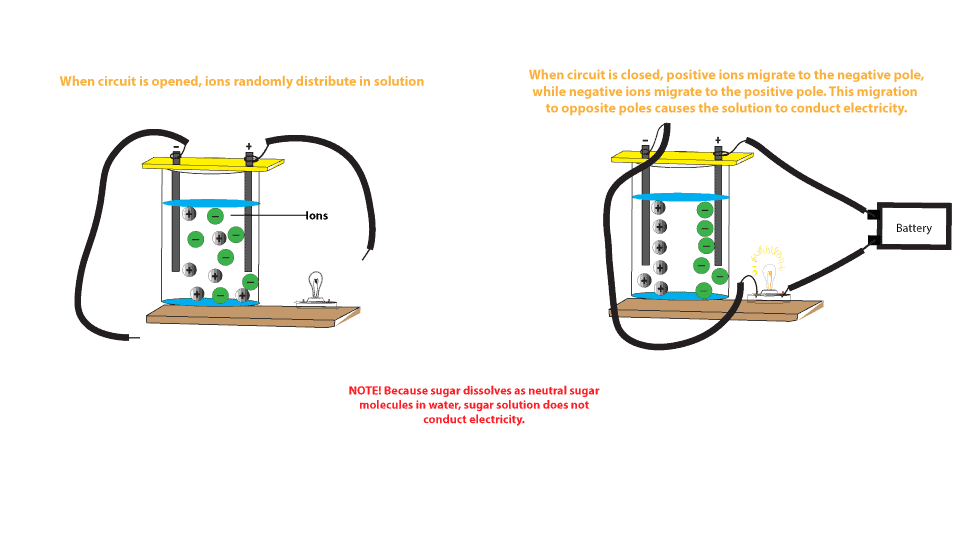
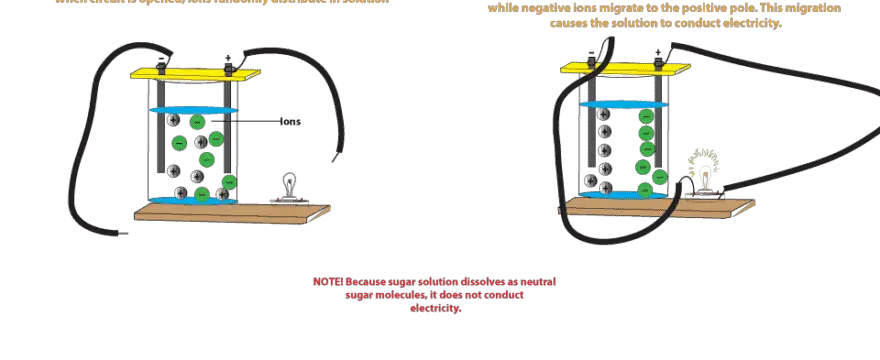
That means ions become seperated and can move easily in solution phase or molten phase. In short salt water can help to produce electricity. To understand why salt water conducts electricity we have to first understand what electricity is. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. These ions are what carry electricity through water with an electric current. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution.
To understand why salt water conducts electricity we have to first understand what electricity is. That means ions become seperated and can move easily in solution phase or molten phase. So solid salts cannot conduct electricity. So it can conduct electricity. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. Electricity is a steady flow of electrons or electrically charged particles through a substance.
 Source: diy.smartkids123.com
Source: diy.smartkids123.com
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. So it can conduct electricity. But salt in aqueous or in molten state has movable ions.
 Source: slideshare.net
Source: slideshare.net
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. Electricity is a steady flow of electrons or electrically charged particles through a substance. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. These ions are what carry electricity through water with an electric current.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
But salt in aqueous or in molten state has movable ions. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. In short salt water can help to produce electricity. To understand why salt water conducts electricity we have to first understand what electricity is. So solid salts cannot conduct electricity.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
But salt in aqueous or in molten state has movable ions. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. Electricity is a steady flow of electrons or electrically charged particles through a substance. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. To understand why salt water conducts electricity we have to first understand what electricity is. So it can conduct electricity. Electricity is a steady flow of electrons or electrically charged particles through a substance. But salt in aqueous or in molten state has movable ions.
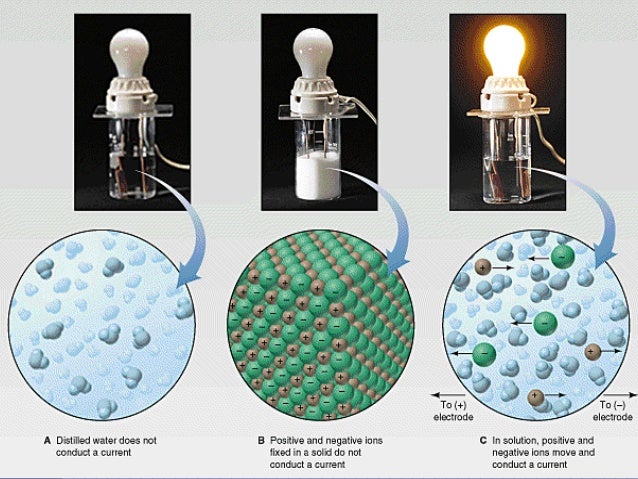 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. So it can conduct electricity. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. To understand why salt water conducts electricity we have to first understand what electricity is.
 Source: slideplayer.com
Source: slideplayer.com
So it can conduct electricity. Electricity is a steady flow of electrons or electrically charged particles through a substance. That means ions become seperated and can move easily in solution phase or molten phase. But salt in aqueous or in molten state has movable ions. So solid salts cannot conduct electricity.
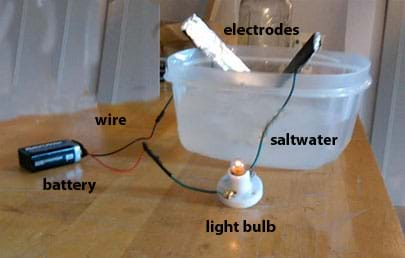 Source: teachengineering.org
Source: teachengineering.org
So it can conduct electricity. In short salt water can help to produce electricity. But salt in aqueous or in molten state has movable ions. When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. These ions are what carry electricity through water with an electric current.
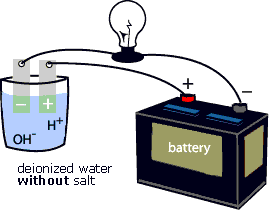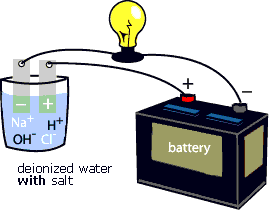 Source: cotf.edu
Source: cotf.edu
Electricity is a steady flow of electrons or electrically charged particles through a substance. These ions are what carry electricity through water with an electric current. So solid salts cannot conduct electricity. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. In short salt water can help to produce electricity.
 Source: otbanvali.over-blog.com
Source: otbanvali.over-blog.com
When you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. These ions are what carry electricity through water with an electric current. So solid salts cannot conduct electricity. To understand why salt water conducts electricity we have to first understand what electricity is.
 Source: nicepng.com
Source: nicepng.com
To understand why salt water conducts electricity we have to first understand what electricity is. Electricity is a steady flow of electrons or electrically charged particles through a substance. These ions are what carry electricity through water with an electric current. In short salt water can help to produce electricity. That means ions become seperated and can move easily in solution phase or molten phase.
 Source: youtube.com
Source: youtube.com
In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. Electricity is a steady flow of electrons or electrically charged particles through a substance. So solid salts cannot conduct electricity. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
 Source: brainly.in
Source: brainly.in
So it can conduct electricity. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. So solid salts cannot conduct electricity. In short salt water can help to produce electricity. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution.
 Source: slideplayer.com
Source: slideplayer.com
To understand why salt water conducts electricity we have to first understand what electricity is. So it can conduct electricity. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. That means ions become seperated and can move easily in solution phase or molten phase.
 Source: pinterest.com
Source: pinterest.com
To understand why salt water conducts electricity we have to first understand what electricity is. So solid salts cannot conduct electricity. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. These ions are what carry electricity through water with an electric current. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title can salt conduct electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.