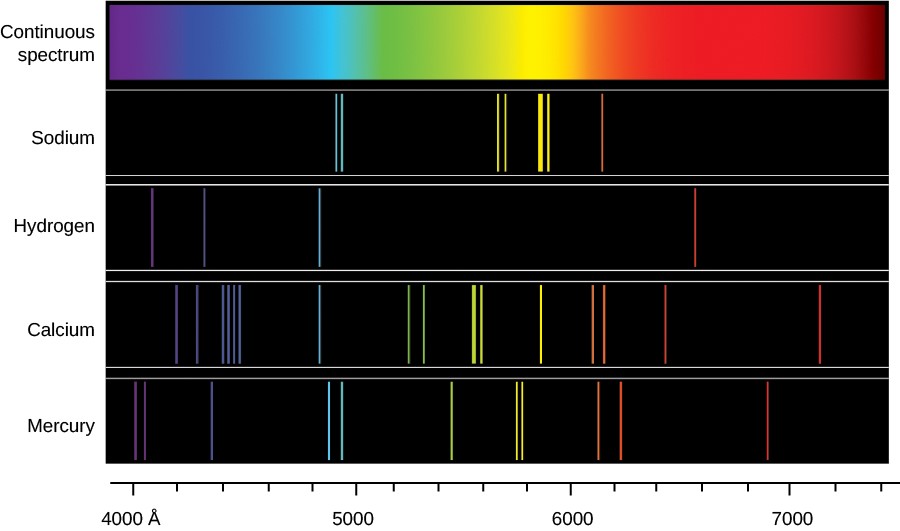
Bright line spectrum
Bright Line Spectrum. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. The emission and absorption spectra of the elements depend on the electronic structure of the atom. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated. An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons.
 Spectroscopy In Astronomy Astronomy From courses.lumenlearning.com
Spectroscopy In Astronomy Astronomy From courses.lumenlearning.com
Continuous spectra contain no observable gaps. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. Spectral lines are often used to identify atoms and molecules. The emission spectrum can be used to determine the composition of a material since it is different for each element of the periodic table. The emission and absorption spectra of the elements depend on the electronic structure of the atom.
These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible.
Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. The production of line spectra by the atoms of an element indicate that an atom can radiate only a certain amount of energy. There are huge gaps between lines. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Continuous spectra contain no observable gaps. They are called brightline or emission spectra.
 Source: physics.smu.edu
Source: physics.smu.edu
Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated. They are called brightline or emission spectra. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background.
 Source: wps.prenhall.com
Source: wps.prenhall.com
Spectral lines are often used to identify atoms and molecules. Spectral lines are often used to identify atoms and molecules. Other articles where line spectrum is discussed. They are called brightline or emission spectra. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated. This produces an absorption spectrum which has dark lines in the same position as the bright lines in the emission spectrum of an element. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. Spectral lines are often used to identify atoms and molecules. They are called brightline or emission spectra.
 Source: chem.libretexts.org
Source: chem.libretexts.org
They are called brightline or emission spectra. They are called brightline or emission spectra. This produces an absorption spectrum which has dark lines in the same position as the bright lines in the emission spectrum of an element. Other articles where line spectrum is discussed. Spectral lines are often used to identify atoms and molecules.
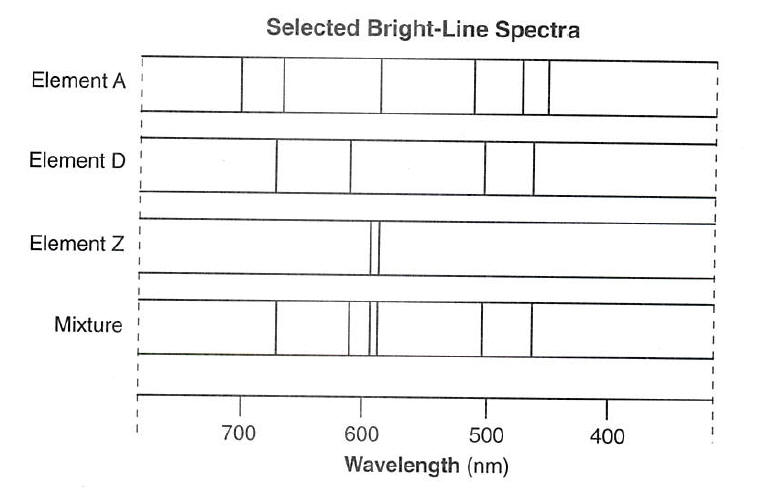 Source: kentchemistry.com
Source: kentchemistry.com
An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. The emission and absorption spectra of the elements depend on the electronic structure of the atom. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. There are huge gaps between lines.
 Source: sas.upenn.edu
Source: sas.upenn.edu
The production of line spectra by the atoms of an element indicate that an atom can radiate only a certain amount of energy. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. This produces an absorption spectrum which has dark lines in the same position as the bright lines in the emission spectrum of an element. Continuous spectra contain no observable gaps. There are huge gaps between lines.
 Source: youtube.com
Source: youtube.com
Other articles where line spectrum is discussed. The emission spectrum can be used to determine the composition of a material since it is different for each element of the periodic table. Other articles where line spectrum is discussed. This leads to the conclusion that bound electrons cannot have just any amount of energy but only a certain amount of energy. The production of line spectra by the atoms of an element indicate that an atom can radiate only a certain amount of energy.
 Source: simple.wikipedia.org
Source: simple.wikipedia.org
An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. Other articles where line spectrum is discussed. This produces an absorption spectrum which has dark lines in the same position as the bright lines in the emission spectrum of an element. The emission spectrum can be used to determine the composition of a material since it is different for each element of the periodic table.
 Source: donklipstein.com
Source: donklipstein.com
An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons. Other articles where line spectrum is discussed. There are huge gaps between lines. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. Continuous spectra contain no observable gaps.
Source: quora.com
Continuous spectra contain no observable gaps. Other articles where line spectrum is discussed. Spectral lines are often used to identify atoms and molecules. An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies.
 Source: pinterest.com
Source: pinterest.com
These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons. The emission and absorption spectra of the elements depend on the electronic structure of the atom. Spectral lines are often used to identify atoms and molecules. They are called brightline or emission spectra.
 Source: pa01001042.schoolwires.net
Source: pa01001042.schoolwires.net
These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Other articles where line spectrum is discussed. This leads to the conclusion that bound electrons cannot have just any amount of energy but only a certain amount of energy. They are called brightline or emission spectra. Spectral lines are often used to identify atoms and molecules.
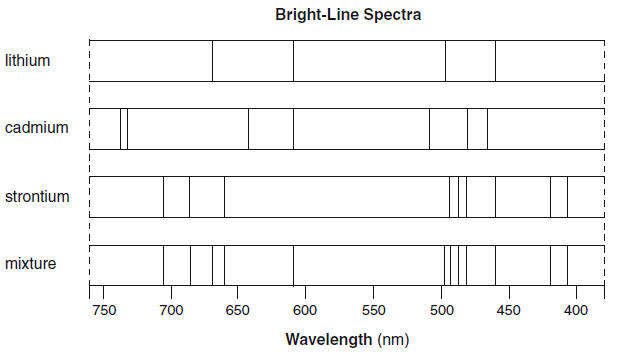 Source: kentchemistry.com
Source: kentchemistry.com
Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated. There are huge gaps between lines. This leads to the conclusion that bound electrons cannot have just any amount of energy but only a certain amount of energy. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Spectral lines are often used to identify atoms and molecules.
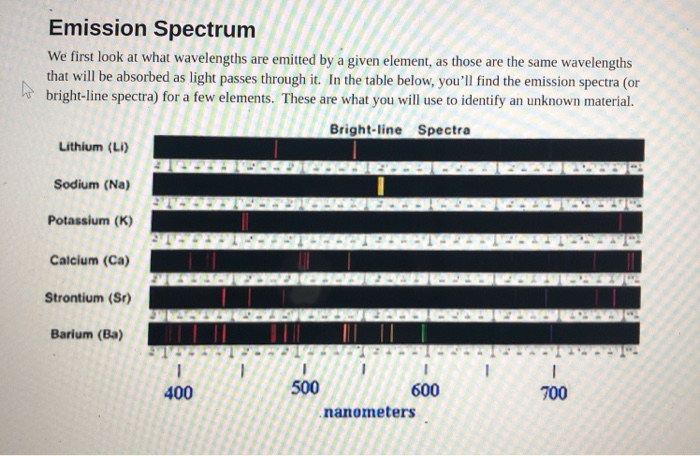 Source: chegg.com
Source: chegg.com
There are huge gaps between lines. The emission spectrum can be used to determine the composition of a material since it is different for each element of the periodic table. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. Spectral lines are often used to identify atoms and molecules. The emission and absorption spectra of the elements depend on the electronic structure of the atom.
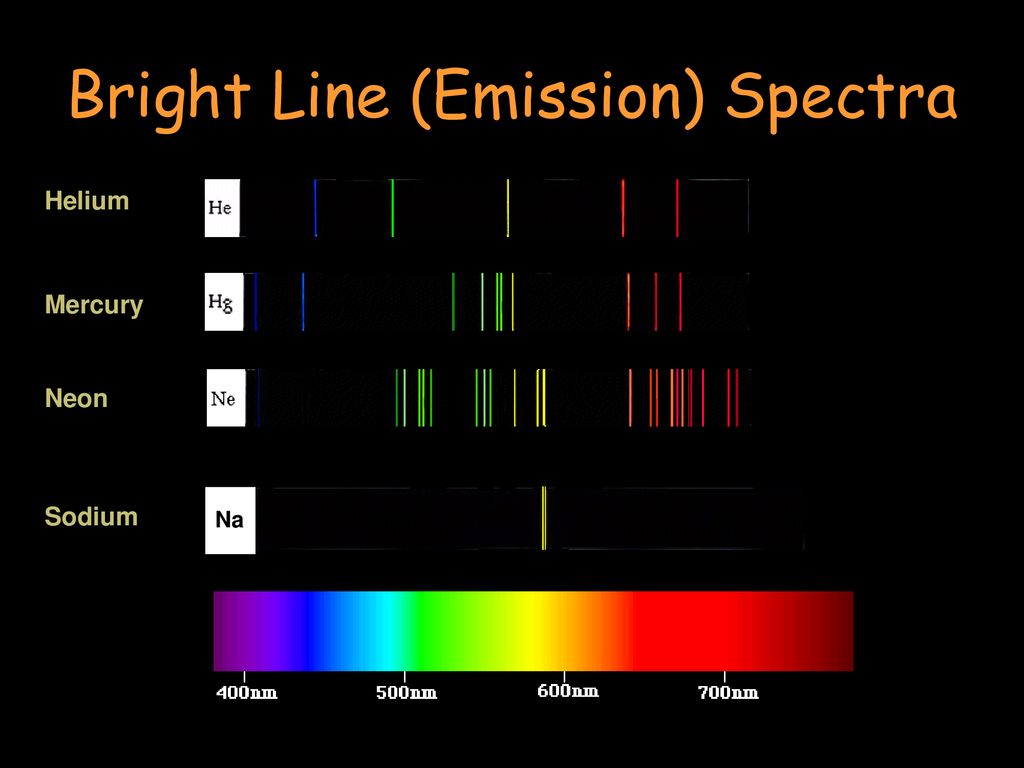 Source: slideplayer.com
Source: slideplayer.com
Other articles where line spectrum is discussed. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. Continuous spectra contain no observable gaps. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation a different kind of spectrum is observed when pure samples of individual elements are heated. An atom consists of a number of negatively charged electrons bound to a nucleus containing an equal number of positively charged protons.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title bright line spectrum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.