Attraction that causes water molecules to stick together
Attraction That Causes Water Molecules To Stick Together. The positive hydrogen ends of one water molecule attract the negative oxygen ends of nearby water molecules causing them to stick together like weak magnets. Water molecules are attracted towards themselves. Movement of electrons changes the attraction between molecules so you can think of them as behaving like magnets. They are called hydrogen bonds.
 Cohesion And Adhesion Water Properties Examples Expii From expii.com
Cohesion And Adhesion Water Properties Examples Expii From expii.com
Attraction between molecules of the same substance. Causes water molecules to draw together. Beads of water on a table surface tension. Water molecules are very polar which causes the molecules to be attracted to each other. Cohesion is the attraction force between molecules of the same substance. Water molecules are attracted towards themselves.
The forces that cause adhesion and cohesion can be divided into several types.
Molecules stick together and don t spread out ex. Cohesion is the attraction force between molecules of the same substance. Since all of the molecules are the same this attraction will be very strong. Adhesion is the tendency of dissimilar particles or surfaces to cling to one another cohesion refers to the tendency of similar or identical particles surfaces to cling to one another. The positive hydrogen ends of one water molecule attract the negative oxygen ends of nearby water molecules causing them to stick together like weak magnets. This attraction force causes the molecules to stick together.
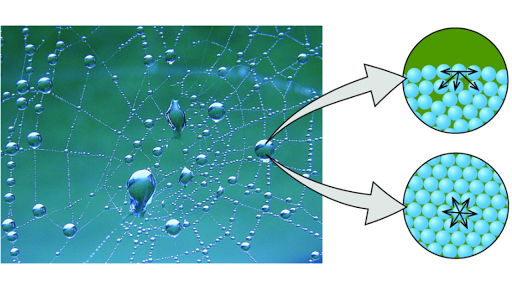
Because the negative end of one molecule s oxygen can form a hydrogen bond with the positive hydrogen of another molecule. A water droplet on a desk for. This attraction force causes the molecules to stick together. This cohesion occurs in water due to hydrogen bonding in water molecules. Beads of water on a table surface tension.
Because of the polarity of water molecules hydrogen bonding occurs between water and other electronegative molecules causing them to stick together why is water cohesive. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of. Why is water adhesive. Certain parts of a molecule will be attracted to other parts of different molecules and will cause them to stick together. This means that the molecules in a rain drop are all attracted to each other and they stick together.
 Source: hectorserranomarinescience.weebly.com
Source: hectorserranomarinescience.weebly.com
This attraction causes water molecules to form temporary bonds that break easily. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of. Because the negative end of one molecule s oxygen can form a hydrogen bond with the positive hydrogen of another molecule. A water droplet on a desk for. This causes the electrons to be unequally distributed causing unequal.
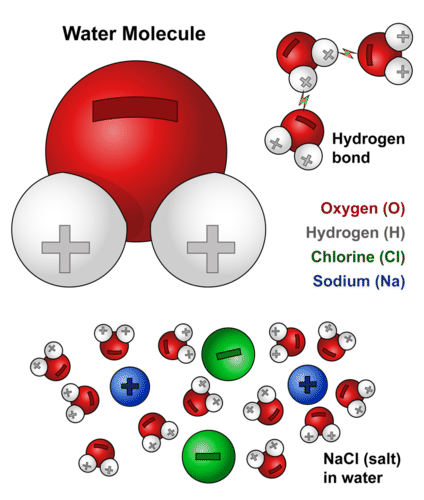
 Source: kayjayr-akshay.blogspot.com
Source: kayjayr-akshay.blogspot.com
Since all of the molecules are the same this attraction will be very strong. Movement of electrons changes the attraction between molecules so you can think of them as behaving like magnets. Why is water adhesive. This cohesion occurs in water due to hydrogen bonding in water molecules. Water molecules are polar.
 Source: manoa.hawaii.edu
Source: manoa.hawaii.edu
They are called hydrogen bonds. Since all of the molecules are the same this attraction will be very strong. Water molecules are attracted towards themselves. It is a mutual attraction between molecules. Because the negative end of one molecule s oxygen can form a hydrogen bond with the positive hydrogen of another molecule.
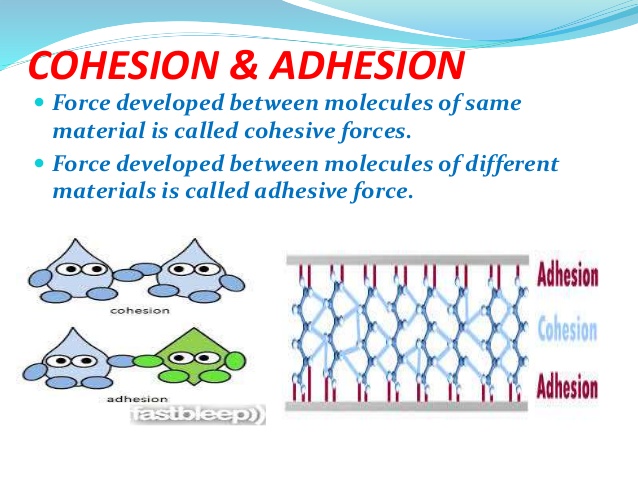
 Source: slideplayer.com
Source: slideplayer.com
Their is another property called adhesive property which causes the water molecules to stick to other surfaces. This attraction causes water molecules to form temporary bonds that break easily. One end is more electronegative than the other. Because the negative end of one molecule s oxygen can form a hydrogen bond with the positive hydrogen of another molecule. Because of the polarity of water molecules hydrogen bonding occurs between water and other electronegative molecules causing them to stick together why is water cohesive.
 Source: expii.com
Source: expii.com
Because of the polarity of water molecules hydrogen bonding occurs between water and other electronegative molecules causing them to stick together why is water cohesive. Certain parts of a molecule will be attracted to other parts of different molecules and will cause them to stick together. Beads of water on a table surface tension. The positive hydrogen ends of one water molecule attract the negative oxygen ends of nearby water molecules causing them to stick together like weak magnets. Ability for water molecules to stick together.
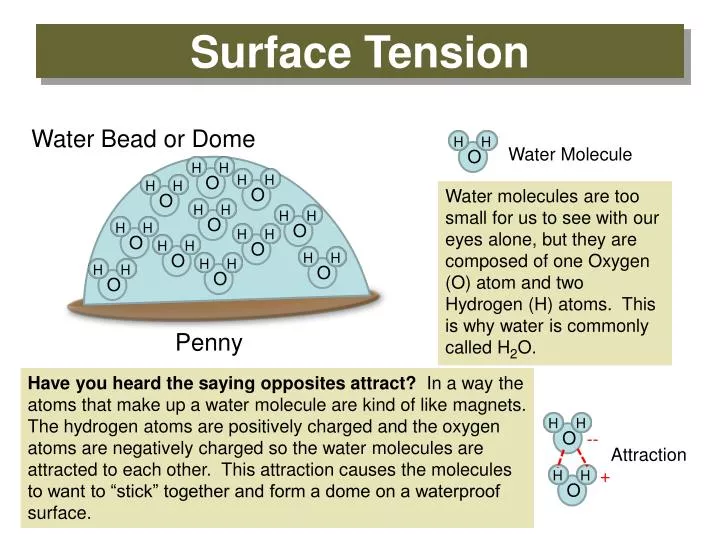 Source: slideserve.com
Source: slideserve.com
Ability for water molecules to stick together. Water molecules are very polar which causes the molecules to be attracted to each other. The positive hydrogen ends of one water molecule attract the negative oxygen ends of nearby water molecules causing them to stick together like weak magnets. Beads of water on a table surface tension. Causes water molecules to draw together.
 Source: usgs.gov
Source: usgs.gov
Beads of water on a table surface tension. Adhesion is the tendency of dissimilar particles or surfaces to cling to one another cohesion refers to the tendency of similar or identical particles surfaces to cling to one another. Cohesion is the attraction force between molecules of the same substance. The forces that cause adhesion and cohesion can be divided into several types. Water molecules are attracted towards themselves.
 Source: sites.google.com
Source: sites.google.com
Such type of sticking is called cohesion. Adhesion is the tendency of dissimilar particles or surfaces to cling to one another cohesion refers to the tendency of similar or identical particles surfaces to cling to one another. Water molecules are attracted towards themselves. Water molecules are very polar which causes the molecules to be attracted to each other. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of.
 Source: usgs.gov
Source: usgs.gov
A water droplet on a desk for. Water molecules are attracted towards themselves. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of. Cohesion is the attraction force between molecules of the same substance. Certain parts of a molecule will be attracted to other parts of different molecules and will cause them to stick together.
 Source: slideplayer.com
Source: slideplayer.com
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another cohesion refers to the tendency of similar or identical particles surfaces to cling to one another. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of. Because of the polarity of water molecules hydrogen bonding occurs between water and other electronegative molecules causing them to stick together why is water cohesive. This cohesion occurs in water due to hydrogen bonding in water molecules. This attraction causes water molecules to form temporary bonds that break easily.
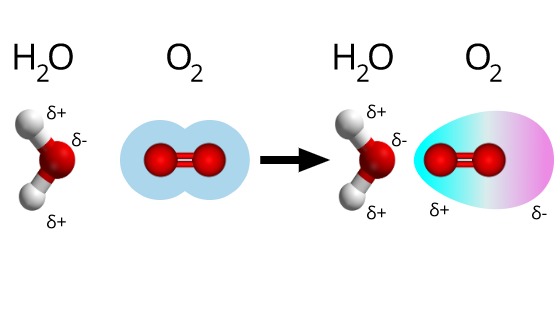 Source: visionlearning.com
Source: visionlearning.com
This cohesion occurs in water due to hydrogen bonding in water molecules. One end is more electronegative than the other. Attraction between molecules of the same substance. Beads of water on a table surface tension. This causes the electrons to be unequally distributed causing unequal.
 Source: manoa.hawaii.edu
Source: manoa.hawaii.edu
They are called hydrogen bonds. Water molecules are polar. Because the negative end of one molecule s oxygen can form a hydrogen bond with the positive hydrogen of another molecule. Movement of electrons changes the attraction between molecules so you can think of them as behaving like magnets. This causes the electrons to be unequally distributed causing unequal.
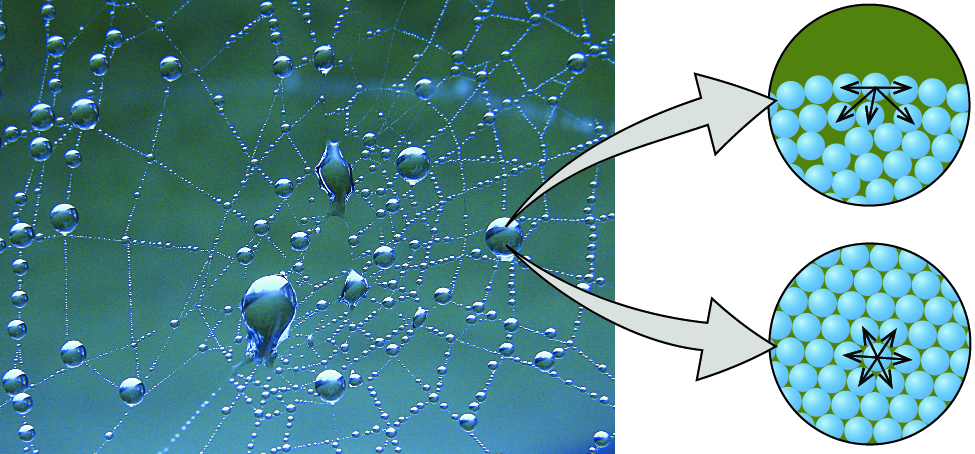 Source: khanacademy.org
Source: khanacademy.org
This causes the electrons to be unequally distributed causing unequal. Cohesive forces are intermolecular forces since these forces can be found between the molecules of the same substance. This attraction force causes the molecules to stick together. Why is water adhesive. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title attraction that causes water molecules to stick together by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





