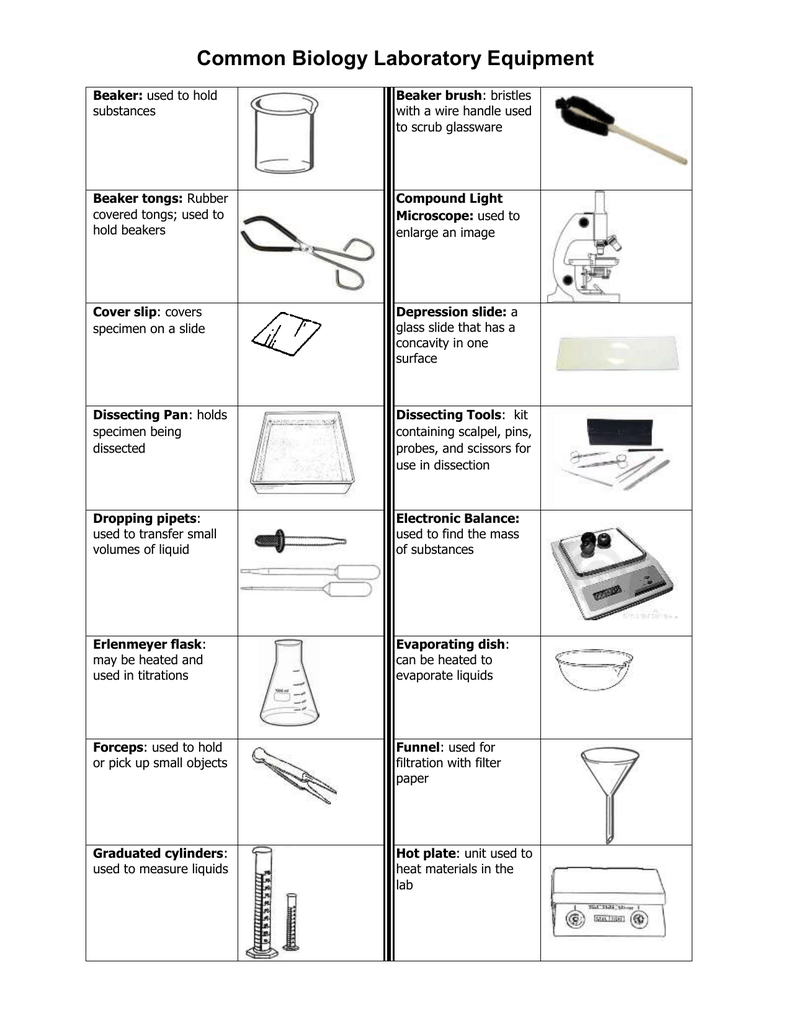
Alloys are important because
Alloys Are Important Because. Strength hardness durability ductility tensile strength and toughness. Alloys are important because. Because of shorter tyre side wall the car inclines less when cornering. Alloys are so important because they enhance the functions of the metal making it stronger or more useful in different situations.
 Lecture 7 3 Metallic Bonds From pt.slideshare.net
Lecture 7 3 Metallic Bonds From pt.slideshare.net
Most important alloys and their useful properties. The most important matter about the light alloy wheels is that other than positive enhancement of criterias such as performance driving comfort economy and visual enhancement that it is a part about your safety which is critical for you and your loved ones lives. Ya what he said. Alloys are important because. Alloys are important because their properties are often superior to those of their component elements. An alloy is a metal composed of more than one element and may have properties different from those of its parent elements.
Alloys can have special properties and can be harder than the original metal more conductive to heat or electricity or less prone to rust and corrosion.
An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. Alloys are made because they contain properties that the pure metal doesn t have which makes them more useful in practical applications. Ya what he said. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. Alloys are important because their properties are often superior to those of their component elements.
 Source: slideserve.com
Source: slideserve.com
Most important alloys and their useful properties. The structure of metals explains their high melting and boiling points and their conductivity. Alloys are important because. The most important matter about the light alloy wheels is that other than positive enhancement of criterias such as performance driving comfort economy and visual enhancement that it is a part about your safety which is critical for you and your loved ones lives. Alloys are made because they contain properties that the pure metal doesn t have which makes them more useful in practical applications.
 Source: quizlet.com
Source: quizlet.com
Strength hardness durability ductility tensile strength and toughness. Alloys can have special properties and can be harder than the original metal more conductive to heat or electricity or less prone to rust and corrosion. The properties of a metal can be modified by mixing it with another substance to form an alloy. Ask question 100. The structure of metals explains their high melting and boiling points and their conductivity.
 Source: slideplayer.com
Source: slideplayer.com
The most important matter about the light alloy wheels is that other than positive enhancement of criterias such as performance driving comfort economy and visual enhancement that it is a part about your safety which is critical for you and your loved ones lives. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. Because of shorter tyre side wall the car inclines less when cornering. Alloys are important because they have properties that differ from those of pure metals.
 Source: slideplayer.com
Source: slideplayer.com
The structure of metals explains their high melting and boiling points and their conductivity. Alloys are important because they have properties that differ from those of pure metals. Most important alloys and their useful properties. Alloys and properties of some common alloys definition. Alloys are made because they contain properties that the pure metal doesn t have which makes them more useful in practical applications.
 Source: slideplayer.com
Source: slideplayer.com
Ya what he said. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. Corrosion resistance ductility hardness and toughness. The structure of metals explains their high melting and boiling points and their conductivity. Many people believe steel is an alloy of iron and nickel but it consists primarily of iron carbon and any of several other metals.
 Source: slideplayer.com
Source: slideplayer.com
Alloys are so important because they enhance the functions of the metal making it stronger or more useful in different situations. Get your answers by asking now. Alloys are important because their properties are often superior to those of their component elements. Corrosion resistance ductility hardness and toughness. Ya what he said.
 Source: slideserve.com
Source: slideserve.com
Example mild steel is 0 1 0 3 carbon and 99 9 99 7 iron. Ya what he said. Alloys and properties of some common alloys definition. Example mild steel is 0 1 0 3 carbon and 99 9 99 7 iron. Alloys are important because they have properties that differ from those of pure metals.
 Source: coursehero.com
Source: coursehero.com
If ice is less dense than liquid water shouldn t it behave as a gas. Many people believe steel is an alloy of iron and nickel but it consists primarily of iron carbon and any of several other metals. The properties of a metal can be modified by mixing it with another substance to form an alloy. Most important alloys and their useful properties. Ya what he said.
 Source: slideserve.com
Source: slideserve.com
If ice is less dense than liquid water shouldn t it behave as a gas. An alloy is a metal composed of more than one element and may have properties different from those of its parent elements. Alloys are important because. Because of shorter tyre side wall the car inclines less when cornering. Many people believe steel is an alloy of iron and nickel but it consists primarily of iron carbon and any of several other metals.
 Source: slideplayer.com
Source: slideplayer.com
An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. Example mild steel is 0 1 0 3 carbon and 99 9 99 7 iron. If ice is less dense than liquid water shouldn t it behave as a gas. Join yahoo answers and get 100 points today. Ya what he said.
 Source: slideplayer.com
Source: slideplayer.com
Alloys are important because their properties are often superior to those of their component elements. The properties of a metal can be modified by mixing it with another substance to form an alloy. Join yahoo answers and get 100 points today. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as.
 Source: slideplayer.com
Source: slideplayer.com
Because of these properties they can be adapted to specific uses where a pure metal would be either unsuitable or cost prohibitive. Alloys are made because they contain properties that the pure metal doesn t have which makes them more useful in practical applications. Alloys are so important because they enhance the functions of the metal making it stronger or more useful in different situations. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. If ice is less dense than liquid water shouldn t it behave as a gas.
 Source: slideserve.com
Source: slideserve.com
Alloys and properties of some common alloys definition. Alloys are so important because they enhance the functions of the metal making it stronger or more useful in different situations. If ice is less dense than liquid water shouldn t it behave as a gas. German silver and tibetan silver are examples of alloys that have the name but don t contain any elemental silver. Many people believe steel is an alloy of iron and nickel but it consists primarily of iron carbon and any of several other metals.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Corrosion resistance ductility hardness and toughness. Ya what he said. Although sterling silver is an alloy consisting mainly of silver many alloys with the word silver in their names are only silver in color. Because of shorter tyre side wall the car inclines less when cornering. Alloys are important because.
 Source: slideshare.net
Source: slideshare.net
An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. An alloy is a metal parent metal combined with other substances alloying agents resulting in superior properties such as. German silver and tibetan silver are examples of alloys that have the name but don t contain any elemental silver. If ice is less dense than liquid water shouldn t it behave as a gas. Alloys are important because.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title alloys are important because by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.