10 pure substances
10 Pure Substances. Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. Order the hotline heavy. Chromatography is the process of separating substances into their individual components. Phases of pure substance.
 Pure Substances From sliderbase.com
Pure Substances From sliderbase.com
A mixture of the phases at equilibrium e g melting vaporization or sublimation. Impure substances have a temperature range over which they melt or boil. The best examples of pure substances are pure elements molecules and compounds. Pure substances have a specific boiling point and a specific melting point. Hydrochloric acid hcl 5. It participates predictably in a chemical reaction.
Crystals in general are pure substances.
Impure substances have a temperature range over which they melt or boil. Impure materials may be mixtures of elements mixtures of compounds or mixtures of elements and compounds. Pure substances have a sharply defined one temperature melting or boiling point. Gold silver water salt etc. Water nitrogen helium and carbon dioxide for example are all pure substances. Impure substances have a temperature range over which they melt or boil.
 Source: toppr.com
Source: toppr.com
Hydrogen gold silver oxygen nitrogen methane carbon monoxide carbon dioxide pure water and table salt sodium chloride. Tin sulfur and diamond are examples of pure substances that are chemical elements. It participates predictably in a chemical reaction. Here are someexamples of pure substances. Just try to realize the facts.
 Source: sciencenotes.org
Source: sciencenotes.org
Here are someexamples of pure substances. Pure substances have a specific boiling point and a specific melting point. 10 examples of pure substances. Just try to realize the facts. For the most part it does not matter which definition you use but if you are asked to give examples of pure substances as part of a homework assignment go with examples that meet the narrow chemical definition.
 Source: slideplayer.com
Source: slideplayer.com
Pure substances have a sharply defined one temperature melting or boiling point. If a substance is pure then chromatography will only produce one substance at the end of the process. Tin sulfur and diamond are examples of pure substances that are chemical elements. The best examples of pure substances are pure elements molecules and compounds. Compressed liquid and saturated liquid.
 Source: thoughtco.com
Source: thoughtco.com
10 examples of pure substances. Tin sulfur and diamond are examples of pure substances that are chemical elements. Pure substances have a sharply defined one temperature melting or boiling point. Gold silver water salt etc. The best examples of pure substances are pure elements molecules and compounds.
 Source: youtube.com
Source: youtube.com
Concerning electrical conductivity the copper used in electrical wiring must be pure. Compressed liquid and saturated liquid. Carbon dioxide co2 6. Vapor gas phase 4. The best examples of pure substances are pure elements molecules and compounds.
 Source: thoughtco.com
Source: thoughtco.com
Tin sulfur and diamond are examples of pure substances that are chemical elements. Examples of pure substances include tin sulfur diamond water pure sugar sucrose table salt sodium chloride and baking soda sodium bicarbonate. The best examples of pure substances are pure elements molecules and compounds. Just try to realize the facts. A pure element or compound contains only one substance with no other substances mixed in.
 Source: sliderbase.com
Source: sliderbase.com
Tin sulfur and diamond are examples of pure substances that are chemical elements. Crystals in general are pure substances. A pure substance consists entirely of one type of atom or compound. Sugar sucrose baking soda sodium bicarbonate ammonia. For the most part it does not matter which definition you use but if you are asked to give examples of pure substances as part of a homework assignment go with examples that meet the narrow chemical definition.
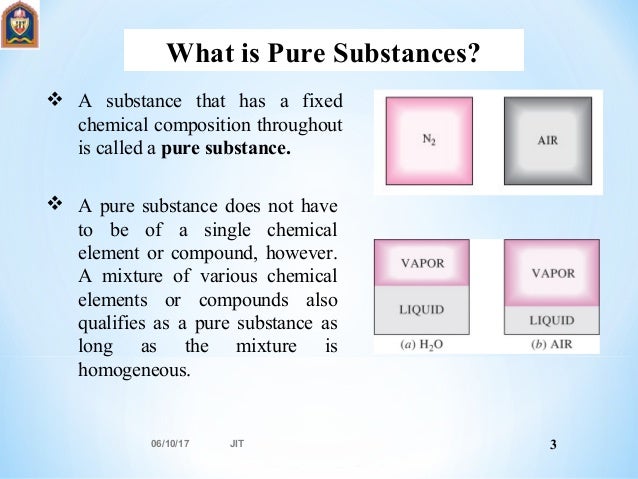 Source: slideshare.net
Source: slideshare.net
Vapor gas phase 4. All elements are pure substances. Order the hotline heavy. Carbon dioxide co2 6. It participates predictably in a chemical reaction.
 Source: toppr.com
Source: toppr.com
It participates predictably in a chemical reaction. Sugar sucrose baking soda sodium bicarbonate ammonia. Pure substances have a sharply defined one temperature melting or boiling point. Hydrogen gold silver oxygen nitrogen methane carbon monoxide carbon dioxide pure water and table salt sodium chloride. 10 examples of pure substances.
 Source: slide-finder.com
Source: slide-finder.com
Just try to realize the facts. Carbon dioxide co2 6. All elements are pure substances. Hydrochloric acid hcl 5. Gold silver water salt etc.
 Source: sciencenotes.org
Source: sciencenotes.org
Chromatography is the process of separating substances into their individual components. It participates predictably in a chemical reaction. 10 examples of pure substances. A material can exist in the 1. Impure substances have a temperature range over which they melt or boil.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Just try to realize the facts. Sugar sucrose baking soda sodium bicarbonate ammonia. Impure materials may be mixtures of elements mixtures of compounds or mixtures of elements and compounds. If a substance is pure then chromatography will only produce one substance at the end of the process. A pure substance consists entirely of one type of atom or compound.
 Source: thoughtco.com
Source: thoughtco.com
Examples of pure substances. A material can exist in the 1. Pure substances have a sharply defined one temperature melting or boiling point. Pure substances have a specific boiling point and a specific melting point. For the most part it does not matter which definition you use but if you are asked to give examples of pure substances as part of a homework assignment go with examples that meet the narrow chemical definition.
 Source: slideshare.net
Source: slideshare.net
Crystals in general are pure substances. Concerning electrical conductivity the copper used in electrical wiring must be pure. Compressed liquid and saturated liquid. 10 examples of pure substances. If a substance is pure then chromatography will only produce one substance at the end of the process.
 Source: researchgate.net
Source: researchgate.net
A pure substance consists entirely of one type of atom or compound. Crystals in general are pure substances. A substance such as pure liquid water is a very poor conductor of electricity due to the lack of electrolytes that assist in the conduction of electricity. Tin sulfur and diamond are examples of pure substances that are chemical elements. Pure substances have a sharply defined one temperature melting or boiling point.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title 10 pure substances by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






